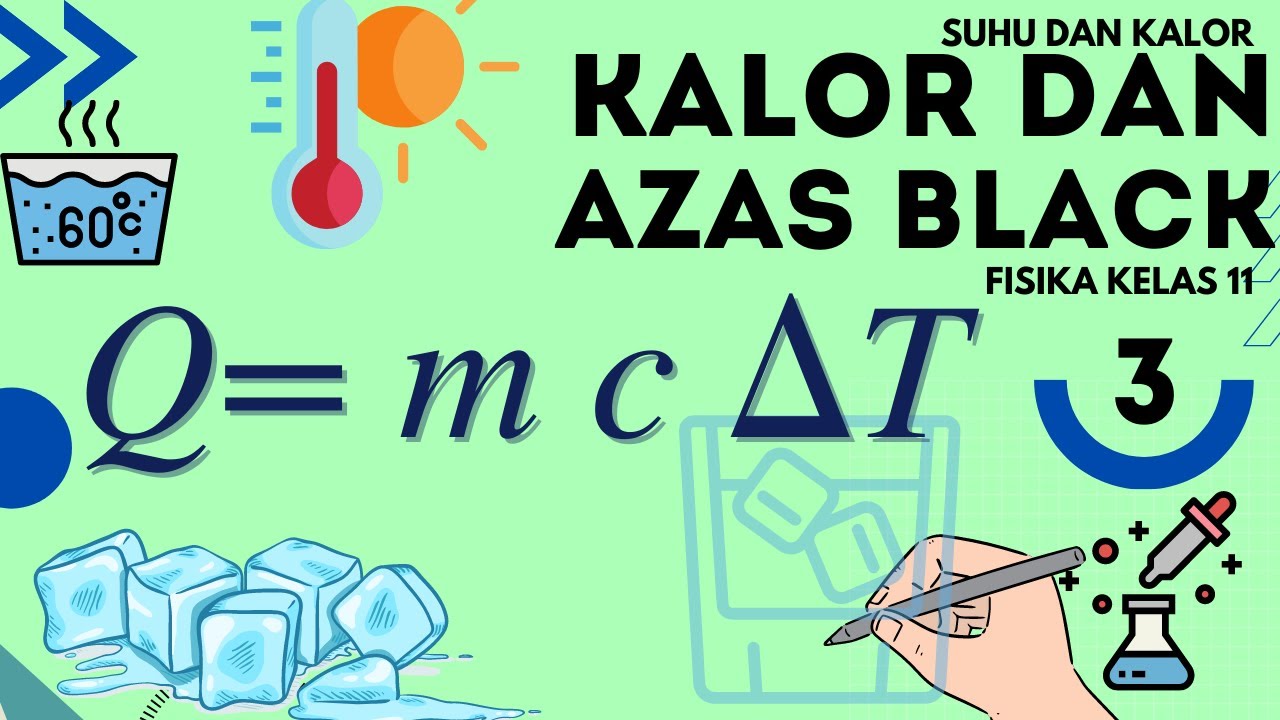TROCAS DE CALOR SEM MUDANÇA DE FASE - TERMOLOGIA - Aula 7 - Prof. Boaro
Summary
TLDRIn this physics lecture, Professor Marcelo Goulart discusses the concept of heat transfer without phase change, focusing on calorimetry and heat exchange between substances. He explains how different materials, such as water, aluminum, and iron, interact thermally in a calorimeter, reaching thermal equilibrium. The session covers key principles like specific heat, the mathematical approach to heat transfer, and the conservation of energy. Through practical examples and equations, students learn how to calculate heat exchange and apply these concepts in real-world scenarios. The lecture also offers guidance on exercises to reinforce learning.
Takeaways
- 😀 The lesson focuses on heat exchange between substances without phase change, emphasizing the role of calorimetry in measuring heat transfer.
- 😀 A calorimeter, often compared to a thermos or coffee jar, is used to prevent heat exchange with the environment, ensuring an isolated system for accurate measurements.
- 😀 The principle of heat exchange in calorimetry is that heat lost by hot substances equals heat gained by cooler substances, reaching thermal equilibrium.
- 😀 The formula for calculating heat exchange is Q = mcΔT, where Q is the heat transferred, m is the mass, c is the specific heat capacity, and ΔT is the temperature change.
- 😀 In calorimetry, the heat gained by substances with lower temperatures and the heat lost by substances with higher temperatures must balance out, with positive heat for heat gained and negative for heat lost.
- 😀 The lesson includes a step-by-step example where water, aluminum, and iron are placed in a calorimeter to demonstrate heat exchange without phase changes.
- 😀 Specific heat capacities vary between substances, which is why different materials like aluminum and water require different amounts of heat to change temperature by the same amount.
- 😀 Practical examples, such as using a thermos or preparing hot coffee, illustrate the real-life application of heat exchange and calorimetry.
- 😀 The capacity of a calorimeter to absorb heat is typically negligible in calculations, as it is made from a combination of materials like plastic, aluminum, and glass.
- 😀 The script concludes with a calculation example involving two substances (water and aluminum) to find the ratio of their specific heat capacities based on heat transfer principles.
Q & A
What is the main focus of the lesson in the video?
-The main focus of the lesson is on heat transfer without a change in phase, specifically using a calorimeter to demonstrate how heat is exchanged between substances until thermal equilibrium is reached.
What is a calorimeter, and how does it work in the context of this lesson?
-A calorimeter is an insulated device used to measure the heat exchange between substances. In this lesson, substances with different temperatures are placed inside the calorimeter, and heat transfer occurs between them until they reach thermal equilibrium, which allows the calculation of the final temperature of the system.
What is the principle of heat exchange in the calorimeter?
-The principle of heat exchange in the calorimeter is based on the idea that heat transferred from warmer substances is absorbed by cooler substances, and the total heat exchanged in the system equals zero, meaning the heat lost by hot substances equals the heat gained by cooler ones.
What is the mathematical formula for calculating heat exchange?
-The formula for calculating heat exchange is Q = mcΔT, where Q is the heat exchanged, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature.
What does the term 'specific heat capacity' refer to in this lesson?
-Specific heat capacity refers to the amount of heat required to raise the temperature of one unit mass of a substance by one degree Celsius. It plays a key role in determining how much heat is exchanged between substances in a calorimeter.
Why is the calorimeter considered ideal in many exercises?
-The calorimeter is considered ideal in many exercises because it is assumed to be perfectly insulated, meaning it does not exchange heat with the environment. In reality, however, this is not the case, as real calorimeters may lose or gain heat, but this effect is often negligible in simplified calculations.
What are the signs used in the heat exchange formula?
-In the heat exchange formula, the sign of Q indicates whether heat is being gained or lost. Heat gained by a substance is positive, while heat lost is negative. This convention helps in balancing the heat exchange equation.
How does the mass of substances affect the heat exchange process?
-The mass of a substance directly affects the amount of heat exchanged. A larger mass requires more heat to change its temperature by a given amount. In the video, this concept is demonstrated with substances like water, aluminum, and iron, each having different masses and specific heat capacities.
What does the term 'thermal equilibrium' refer to?
-Thermal equilibrium refers to the state in which two or more substances in contact no longer exchange heat because they have reached the same temperature. At this point, the heat gained by one substance equals the heat lost by another.
What is the purpose of the example involving water, aluminum, and iron in the calorimeter?
-The purpose of the example is to illustrate how different substances with different initial temperatures interact in a calorimeter. The example demonstrates how the heat exchanged between the substances is calculated, with the goal of reaching thermal equilibrium and determining the final temperature of the system.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)