KONSEP MOL - KIMIA - MATERI UTBK SBMPTN DAN SIMAK UI - UTBK 2022 | SIMAK UI 2022
Summary
TLDRThis educational video, presented by Katty, explores the fundamental concept of mol in chemistry, diving into its importance in stoichiometry. The video explains how to calculate the amount of substances involved in chemical reactions, including methods for determining moles, mass, and volume. It covers the relationship between moles and gas volumes through ideal gas equations, introduces standard conditions like STP and RTP, and explains concentration and molarity in solutions. Katty provides a thorough yet accessible overview, aiming to help viewers understand and apply stoichiometry concepts in real-world scenarios.
Takeaways
- 😀 Stoichiometry is the quantitative aspect of chemistry that involves calculating the amount of substances in a chemical reaction.
- 😀 The mole concept (mol) is the foundation of stoichiometry, used to determine the amount of substances involved in reactions.
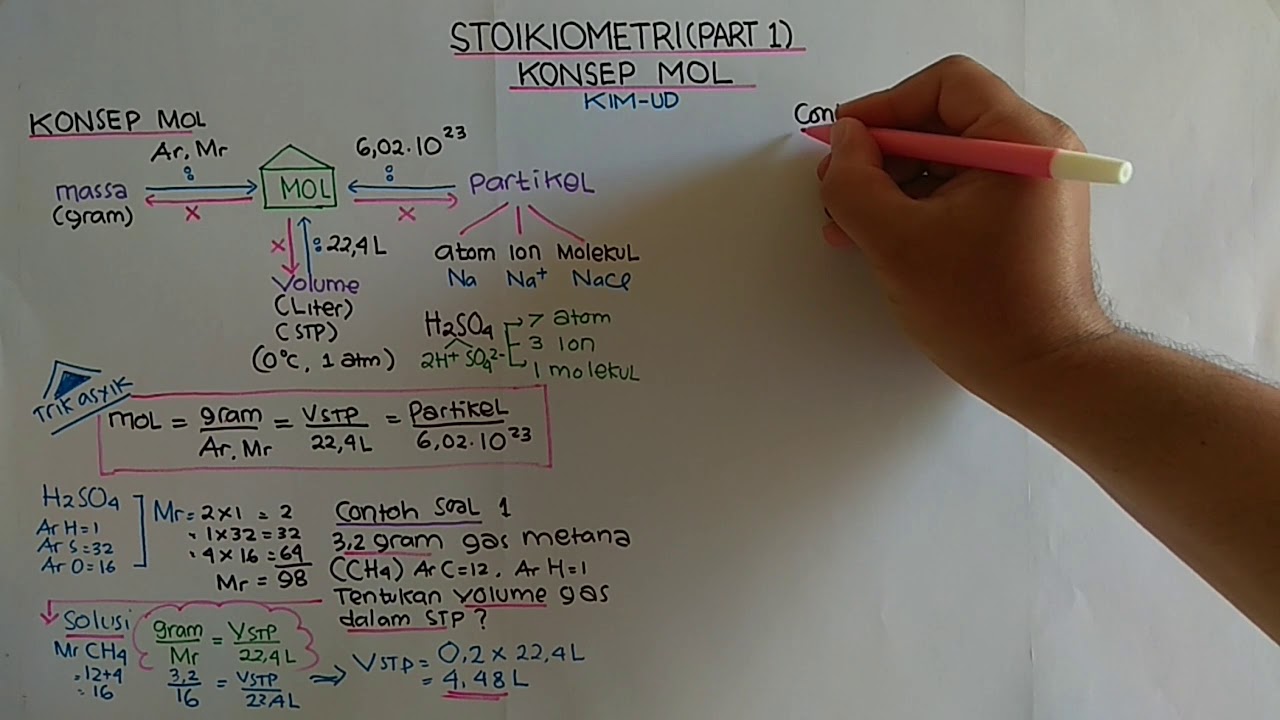
- 😀 The international unit for measuring the amount of substance is the mole (mol), which can be calculated using mass (g) divided by molecular mass (MR).
- 😀 The mole can also be calculated by dividing the number of particles by Avogadro's constant (6.02 x 10^23).
- 😀 Stoichiometry involves calculating the relationship between reactants and products, such as how much of substance A is needed to produce substance C.
- 😀 The relationship between mol and volume can be derived using the ideal gas law: PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature.
- 😀 In special conditions (Standard Temperature and Pressure - STP), 1 mole of gas occupies 22.4 liters, simplifying volume calculations.
- 😀 Room Temperature and Pressure (RTP) is another special condition where 1 mole of gas occupies 24 liters at 25°C and 1 ATM pressure.
- 😀 For two gases measured at the same temperature and pressure, the volume of the gases is directly proportional to the number of moles, which is expressed by the equation V1/n1 = V2/n2.
- 😀 Concentration, or molarity, refers to the amount of solute in a solution, and it is calculated as moles of solute per liter of solution (mol/L).
- 😀 Molarity can also be calculated using density and percentage concentration when those values are known, with the formula: molarity = (density × percentage) / molecular mass.
Q & A
What is stoichiometry in chemistry?
-Stoichiometry is the quantitative aspect of chemistry, where the amounts of substances involved in chemical reactions are calculated. It focuses on determining the amount of reactants and products in a chemical reaction.
What is the significance of the mole in stoichiometry?
-The mole (mol) is a fundamental unit in stoichiometry, as it allows chemists to quantify the amount of a substance based on its atomic or molecular composition, making calculations in chemical reactions possible.
How do you calculate the number of moles of a substance?
-You can calculate the number of moles by dividing the mass of the substance (in grams) by its molar mass (Mr). Alternatively, you can divide the number of particles (atoms, molecules, or ions) by Avogadro's constant (6.022 x 10²³).
What is the relationship between moles and volume of a gas?
-The relationship between moles and volume of a gas is described by the ideal gas law, PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
What does STP (Standard Temperature and Pressure) represent in gas volume calculations?
-At STP, a gas is measured at 1 atmosphere of pressure and 0°C. Under these conditions, 1 mole of any ideal gas occupies 22.4 liters of volume.
What is RTP (Room Temperature and Pressure), and how does it relate to gas volume?
-RTP refers to conditions where the pressure is 1 atmosphere and the temperature is 25°C. Under these conditions, 1 mole of gas occupies 24 liters of volume.
How can molarity be calculated in a solution?
-Molarity is calculated by dividing the number of moles of solute by the volume of the solution in liters. The formula is Molarity = moles of solute / volume of solution (L).
How do you find the molarity if the density and percentage concentration of a substance are given?
-If the density and percentage concentration are known, you can calculate molarity using the formula: Molarity = (Density × Percentage) / Molar mass, where density is in g/L, and percentage is the mass percentage of the solute.
What are the units for molarity, and why is volume measured in liters?
-The units for molarity are moles per liter (mol/L). Volume is measured in liters because it aligns with the standard SI units, and this makes calculations easier in the context of stoichiometry.
How is Avogadro's constant used to relate particles to moles?
-Avogadro's constant (6.022 x 10²³ particles per mole) allows chemists to convert the number of particles (atoms, molecules, ions) into moles, which can then be used in stoichiometric calculations.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)