CONTROL VOLUMES - Closed vs. Open - Extensive vs. Intensive in 9 Minutes!
Summary
TLDRThis video script explores fundamental concepts in thermodynamics, particularly focusing on system classifications such as closed, open, and isolated systems. It highlights the distinctions between these systems using relatable examples, like a fridge, fan, water heater, and thermos. The script also delves into properties like extensive vs. intensive, the significance of 'specific' properties, and the role of control volumes in analyzing systems. It concludes with practical examples to help understand the application of these concepts in more complex systems, with the promise of more detailed discussions in future lectures.
Takeaways
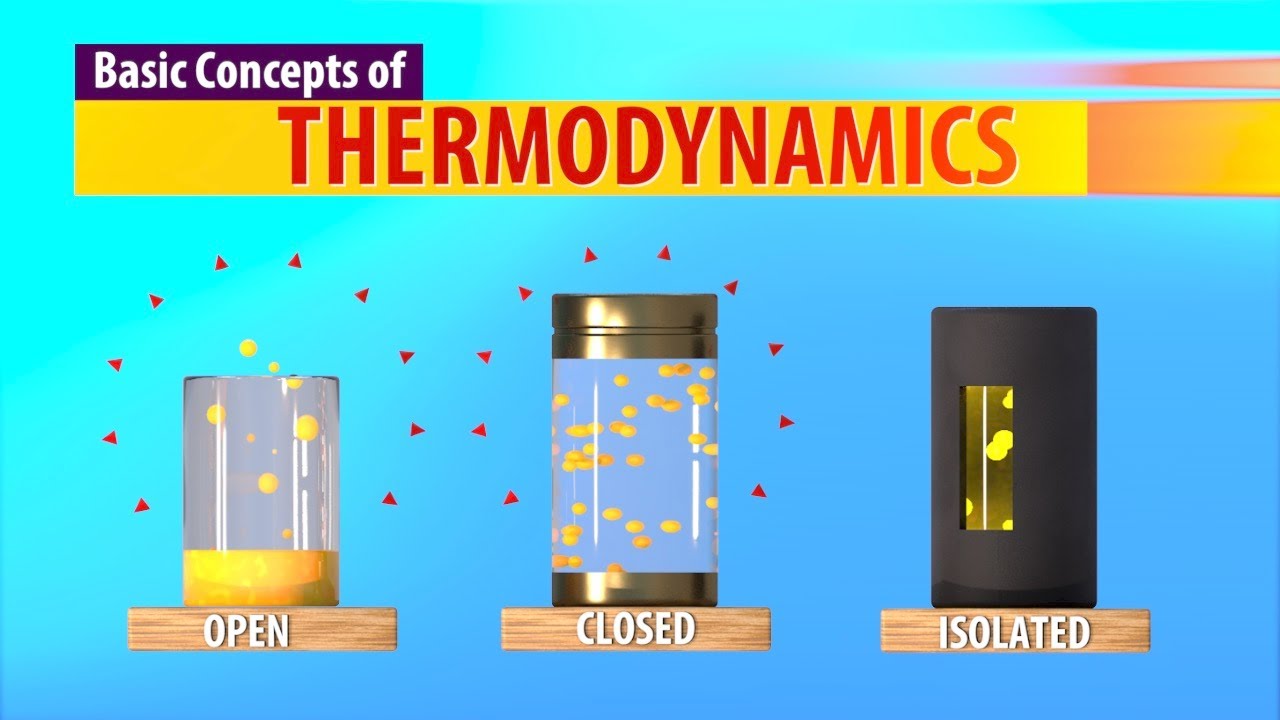
- 😀 Closed systems allow energy exchange but no mass exchange (e.g., closed piston-cylinder).
- 😀 Isolated systems do not exchange mass or energy with their surroundings.
- 😀 Open systems involve both mass and energy exchange with their surroundings (e.g., turbines, pistons with inlets).
- 😀 A control volume is a defined region in space around a system where properties are measured, regardless of volume changes.
- 😀 A system's state is defined by its properties, and a change in properties signifies a process.
- 😀 A process can be represented as a curve or line on a graph showing the transition from one state to another.
- 😀 Equilibrium occurs when a system's properties do not change over time.
- 😀 Iso processes refer to situations where one property remains constant, such as isothermal (constant temperature) and isobaric (constant pressure) processes.
- 😀 Extensive properties depend on the system's size (e.g., volume, mass, energy), while intensive properties are independent of size (e.g., temperature, density).
- 😀 Specific properties are defined as per unit mass (e.g., specific volume, specific heat, specific energy), and molar specific properties are per mole or kilomole.
- 😀 Heat and work are denoted by capital letters for total quantities (e.g., Q for total heat) and lowercase letters with a dot for flow rates (e.g., q̇ for heat flow per unit mass per second).
Q & A
What defines a closed system in thermodynamics?
-A closed system in thermodynamics is defined as a system where no mass exchange occurs, but energy exchange can happen in the form of heat, electricity, or mechanical work.
What is an isolated system?
-An isolated system is a special case of a closed system where neither mass nor energy is exchanged with the surroundings.
What is the difference between open and closed systems?
-In open systems, both mass and energy can flow into or out of the system, whereas in closed systems, only energy can be exchanged, and no mass enters or leaves.
What is a control volume in thermodynamics?
-A control volume is a defined region in space or a system boundary used to analyze thermodynamic processes. The control volume can be static or dynamic, with the boundaries sometimes changing depending on the system's movement or changes.
How are the properties of a system used in thermodynamics?
-The properties of a system, such as temperature, pressure, and volume, define its state. Changes in these properties indicate that the system has undergone a process, which can be visualized as a trajectory on a 2D plot.
What does the prefix 'iso' mean in thermodynamics?
-The prefix 'iso' indicates that a particular property is constant during a process. For example, 'isothermal' means constant temperature, and 'isobaric' means constant pressure.
What is the difference between extensive and intensive properties?
-Extensive properties depend on the size or amount of the system, such as mass and volume. Intensive properties, like temperature and density, are independent of the system's size.
What is a specific property in thermodynamics?
-A specific property refers to a property per unit mass of the system. For instance, specific volume is the volume per unit mass, and specific energy is the energy per unit mass.
What is the significance of using lower case variables for specific properties?
-Lower case variables are used for specific properties to distinguish them from total properties. For example, 'v' represents specific volume (volume per unit mass), while 'V' represents total volume.
How is the molar specific volume calculated?
-The molar specific volume is calculated by multiplying the specific volume (volume per unit mass) by the molecular weight of the substance (kg/kmol), giving the volume per kilomole.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Hukum Termodinamika, Bagian 1: Energi Dalam dan Hukum Pertama

Termodinamika Kelas XI IPA
Basic Concepts of Thermodynamics (Animation)

Lesson 1: Introduction to Thermodynamics (with Mountain Dew)

Sistem Pada Termodinamika dan Contohnya | Sistem Terbuka, Sistem Tertuttup dan Sistem Terisolasi

Termodinamika - Fisika Kelas 11 (Kurikulum 2013 Revisi) - Quipper Video
5.0 / 5 (0 votes)