REAKSI TERMOKIMIA
Summary
TLDRIn this educational video, Upin from 'Putih Aktifnya' channel introduces the basics of thermochemistry, exploring the energy changes involved in chemical reactions. The video explains key concepts such as the difference between systems and surroundings, types of systems (open, closed, and isolated), and the role of enthalpy in reactions. Upin discusses exothermic and endothermic reactions, highlighting how energy is either released or absorbed during chemical processes. The video is a clear and engaging introduction to thermochemistry, with practical examples and visual aids to enhance understanding.
Takeaways
- 😀 Thermochemistry studies the heat or energy changes that occur during chemical reactions.
- 😀 A system refers to the subject of study, while the environment is everything outside the system.
- 😀 Energy can either be absorbed or released during a reaction, transferring between the system and its surroundings.
- 😀 There are three types of systems in thermochemistry: open, closed, and isolated systems.
- 😀 In an open system, both heat and material can be exchanged between the system and the environment.
- 😀 In a closed system, only heat can be exchanged with the environment, but not material.
- 😀 In an isolated system, neither heat nor material is exchanged with the environment.
- 😀 Enthalpy represents the total energy in the form of heat within a system, though only its change (ΔH) can be determined.
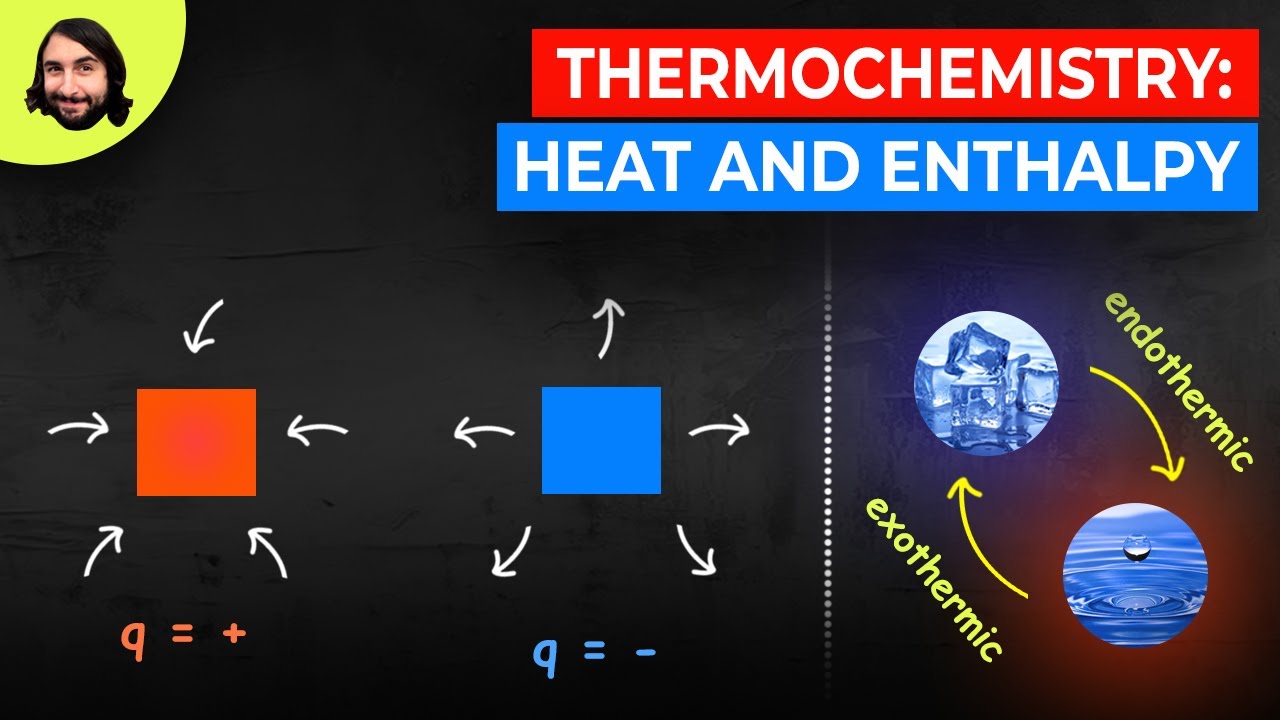
- 😀 Exothermic reactions release heat from the system to the surroundings, causing the surroundings to increase in temperature (ΔH < 0).
- 😀 Endothermic reactions absorb heat from the surroundings, causing the system to cool down (ΔH > 0).
- 😀 Diagrams of exothermic and endothermic reactions show the change in enthalpy: exothermic reactions are represented by a decrease, and endothermic reactions by an increase in enthalpy.
Q & A
What is thermochemistry?
-Thermochemistry is a branch of chemistry that studies the energy changes, specifically the heat (calor) absorbed or released during chemical reactions.
What are the key elements of thermochemistry?
-Thermochemistry focuses on energy changes that occur during chemical reactions, including both the heat absorbed and released by the system.
What is the difference between a system and the environment in thermochemistry?
-In thermochemistry, the system refers to the specific matter or process being studied, while the environment includes everything outside of the system that could interact with it.
What are the three types of systems in thermochemistry?
-The three types of systems are: open systems (which exchange both heat and matter with the surroundings), closed systems (which exchange heat but not matter), and isolated systems (which exchange neither heat nor matter with the surroundings).
Can you give an example of an open system?
-An example of an open system is a boiling kettle, where both heat and steam (matter) are exchanged with the surroundings.
What is enthalpy in thermochemistry?
-Enthalpy is the total heat energy contained in a system. It is the sum of the internal energy of the system and the product of pressure and volume.
How is enthalpy change (ΔH) calculated?
-Enthalpy change (ΔH) is calculated by subtracting the enthalpy of reactants from the enthalpy of products. This is represented by the formula ΔH = H(products) - H(reactants).
What is an exothermic reaction?
-An exothermic reaction is a chemical reaction that releases heat to the surroundings, causing an increase in the temperature of the environment. The enthalpy change (ΔH) for such reactions is negative.
What is an endothermic reaction?
-An endothermic reaction is a chemical reaction that absorbs heat from the surroundings, causing a decrease in the temperature of the environment. The enthalpy change (ΔH) for such reactions is positive.
How can you visually represent the difference between exothermic and endothermic reactions?
-An exothermic reaction can be represented by a graph showing a decrease in energy (ΔH negative), while an endothermic reaction shows an increase in energy (ΔH positive), with the reaction enthalpy increasing as you move from reactants to products.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)