Boyle's Law Practice Problems
Summary
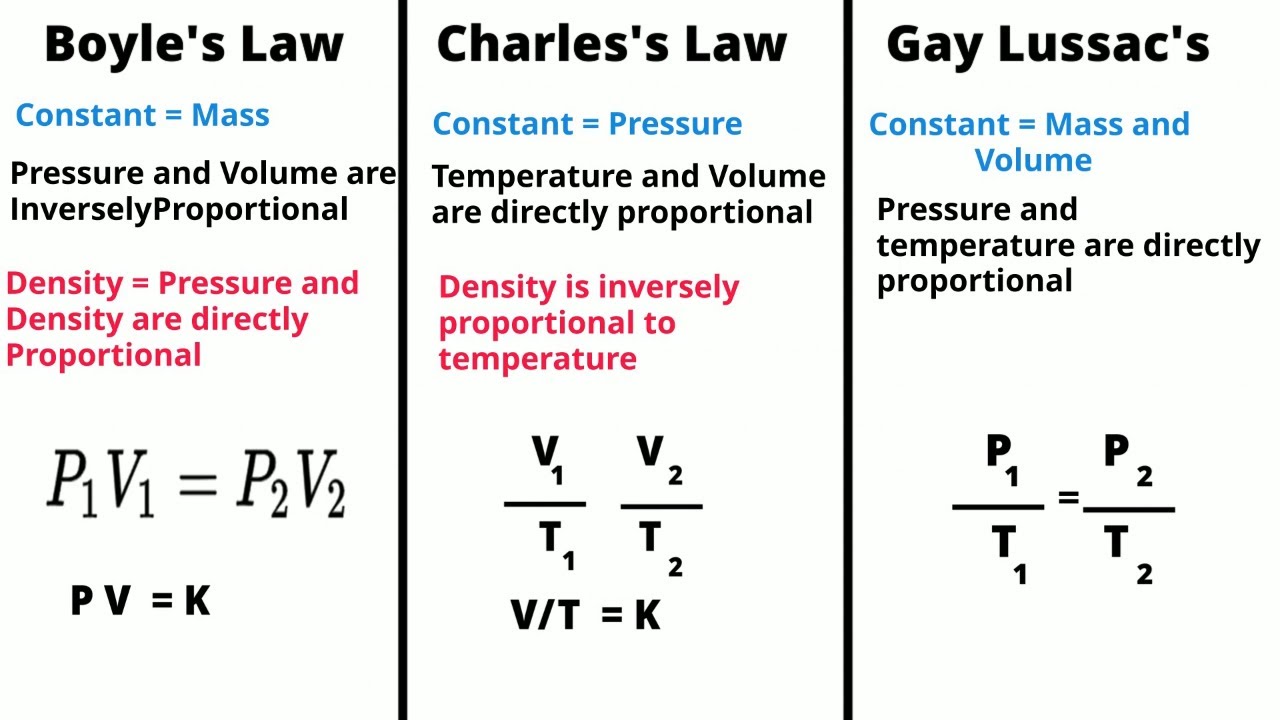
TLDRThis video explains Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume at constant temperature. Through visual illustrations, it demonstrates how reducing volume increases pressure due to more frequent collisions of gas particles with container walls. The law is mathematically represented by the equation P1 × V1 = P2 × V2, and the graph of pressure versus volume forms a hyperbola. Practical problems are solved, showcasing real-world applications of Boyle's Law, including conversions between different pressure units and calculations of gas behavior in varying conditions.
Takeaways
- 😀 Boyle's Law states that the pressure of a gas is inversely related to its volume when temperature is held constant.
- 😀 Reducing the volume of a gas increases the frequency of particle collisions, resulting in higher pressure.
- 😀 The equation representing Boyle's Law is P1 × V1 = P2 × V2, where P represents pressure and V represents volume.
- 😀 The graph of Boyle's Law shows a hyperbolic curve, indicating that as volume increases, pressure decreases.
- 😀 It's important to ensure that pressure measurements are in the same units before applying Boyle's Law.
- 😀 Gas pressure can be expressed in different units such as atm, kPa, or torr; conversions may be necessary.
- 😀 Example calculations demonstrate how to apply Boyle's Law to find unknown pressures and volumes.
- 😀 Understanding Boyle's Law is essential for predicting gas behavior in scientific and practical applications.
- 😀 The relationship described by Boyle's Law is foundational for many areas of chemistry and physics.
- 😀 Consistent units and proper conversions are crucial for accurate calculations in gas laws.
Q & A
What is Boyle's Law?
-Boyle's Law states that the pressure of a gas is inversely proportional to its volume when the temperature is held constant.
How does compression affect gas pressure in a container?
-When a gas container is compressed, reducing its volume, the pressure inside the container increases due to more frequent collisions of gas particles with the container walls.
What equation represents Boyle's Law?
-The equation associated with Boyle's Law is P1 × V1 = P2 × V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
What is the graphical representation of Boyle's Law?
-The graph of Boyle's Law shows pressure on the y-axis and volume on the x-axis, typically exhibiting a hyperbolic curve where pressure decreases as volume increases.
In the first example problem, what was the initial and final pressure when the volume decreased?
-In the first problem, the initial pressure was 4.6 atm, and after decreasing the volume from 2.5 liters to 1.6 liters, the new pressure calculated was approximately 7.19 atm.
Why is it necessary to match units when applying Boyle's Law?
-It is essential to match the units of pressure when applying Boyle's Law to ensure accurate calculations, as different units (like atm, kPa, torr) can yield different numerical results if not converted properly.
How do you convert kPa to torr in the second example problem?
-To convert kPa to torr, you can use the conversion factor where 101.3 kPa equals 760 torr, allowing for direct conversion between the two units.
What was the initial pressure and volume in the second example problem, and what was the final volume needed to achieve a specified pressure?
-In the second problem, the initial pressure was 115 kPa and the volume was 3.5 liters. To decrease the pressure to 625 torr, the final volume needed to be approximately 4.83 liters.
What unit conversion was performed in the third example problem involving psi?
-In the third example, the pressure was initially given in psi (17.5 psi) and was converted to atm using the conversion factor of 14.7 psi per atm to find the final pressure.
What was the final pressure in atm calculated from the third problem?
-The final pressure calculated from the third problem was approximately 2.86 atm after converting from 42 psi.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)