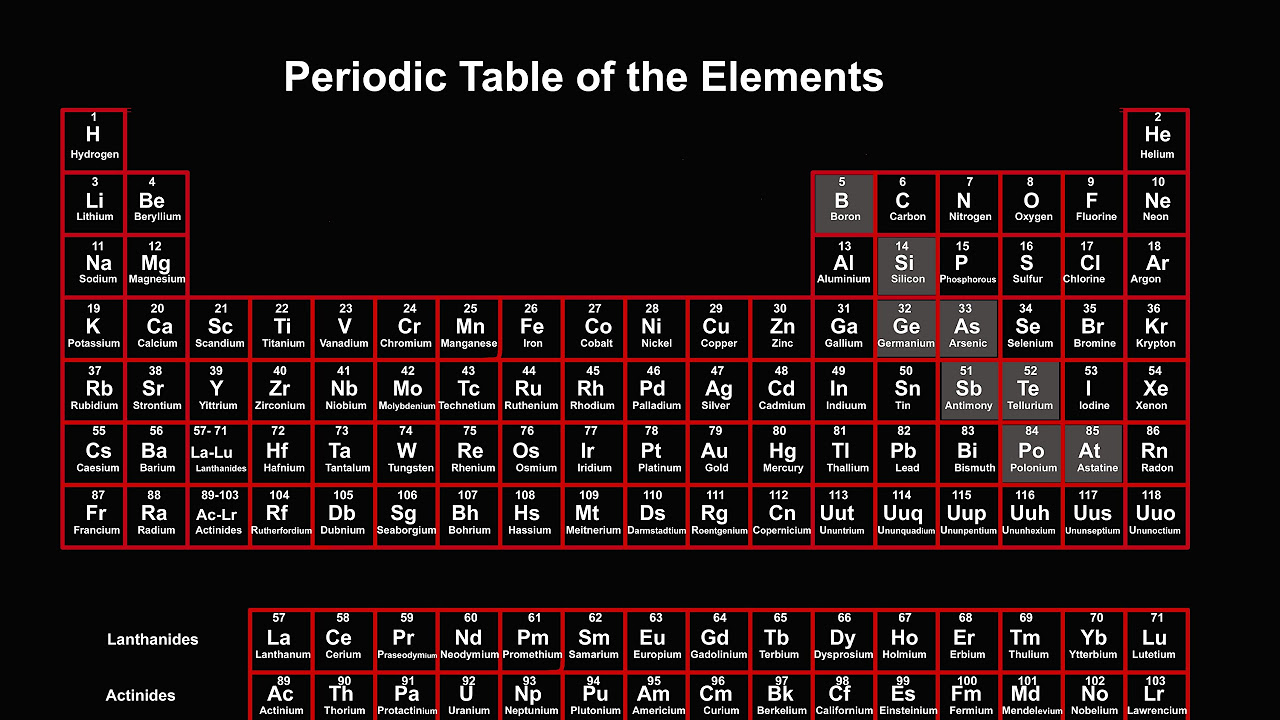
This Element Doesn't Fit the Periodic Table
Summary
TLDRThis SciShow video delves into the unique positioning of hydrogen in the periodic table, highlighting the ongoing debate among chemists about where it truly belongs. Despite being the simplest element, hydrogen exhibits properties that align it with alkali metals, halogens, and even carbon, creating a challenge in categorization. The video emphasizes the importance of the periodic table as a predictive tool for understanding chemical behavior and reactions. Ultimately, it celebrates hydrogen's distinctive qualities while inviting viewers to explore its complexities through interactive learning on the Brilliant platform.
Takeaways
- 😀 Hydrogen's placement in the periodic table is debated among chemists due to its unique properties.
- 🤔 While hydrogen is often grouped with alkali metals, it exhibits behaviors characteristic of multiple groups.
- 🔬 The periodic table is a predictive tool that organizes elements based on their atomic number and chemical properties.
- ⚛️ Atomic number represents the number of protons and electrons in an atom, influencing its behavior in chemical reactions.
- 🧪 Electrons occupy specific patterns or subshells, which repeat as new energy levels are filled.
- 🌌 Hydrogen has a single electron in a shell that can hold two, making it similar to alkali metals like lithium and sodium.
- 🔄 Hydrogen can form positive ions (H⁺) like alkali metals but is not a metal itself.
- 🧪 Hydrogen can also gain an electron to form hydride ions (H⁻), similar to halogens, but differs in electronegativity.
- 💧 Hydrogen, fluorine, and chlorine are gases at room temperature and exist in diatomic form (e.g., H₂, F₂).
- 🎓 A well-constructed periodic table helps predict element behaviors, but misplacing hydrogen can lead to incorrect predictions.
Q & A
Why is hydrogen's position in the periodic table considered controversial?
-Hydrogen's position is controversial because it exhibits properties similar to multiple groups, including alkali metals, halogens, and group 14 elements, but does not fit neatly into any one category.
What is the significance of atomic number in the periodic table?
-Atomic number, which represents the number of protons in an atom, is crucial as it determines the element's position in the periodic table and its chemical properties.
How do electron configurations relate to the organization of the periodic table?
-Elements are organized in the periodic table based on their electron configurations, which dictate how they behave chemically. Elements with similar configurations are grouped together.
In what ways does hydrogen behave like alkali metals?
-Hydrogen can lose its single outer electron to form a positive ion (H+), similar to alkali metals like sodium and lithium, which also tend to lose their outermost electron.
What distinguishes hydrogen from alkali metals?
-Unlike alkali metals, hydrogen is not a metal under normal conditions, and its chemical behavior can differ significantly, such as forming different compounds when reacting with the same elements.
How does hydrogen's electronegativity compare to that of fluorine?
-Fluorine is the most electronegative element, meaning it readily attracts electrons, while hydrogen is better at sharing electrons, which influences its bonding behavior.
What are the potential classifications of hydrogen in the periodic table?
-Chemists propose several classifications for hydrogen, including placement with alkali metals, halogens, or even in group 14, but no single classification is universally accepted.
Why is it important to accurately place elements in the periodic table?
-Accurate placement is vital because it allows scientists to make reliable predictions about an element's behavior and reactions based on its group and position in the table.
What implications does the debate over hydrogen's placement have for chemistry?
-The debate underscores the complexity of elemental properties and the need for a well-structured periodic table to aid in chemical understanding and predict reactions accurately.
What resources does Brilliant offer for those interested in learning more about chemistry?
-Brilliant provides interactive courses in science, including chemistry, that help users understand concepts like molecular structures and chemical behavior through engaging lessons.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)