Protein Folding Mechanism
Summary
TLDRThis video explains the essential biological process of protein folding, highlighting how proteins achieve their functional three-dimensional structures. The process involves converting a nascent, linear polypeptide chain into a functional native protein. The stages of folding, from primary to tertiary (and sometimes quaternary) structures, are discussed, along with how hydrophilic and hydrophobic interactions shape the final form. The role of molecular chaperones in correcting misfolded proteins and preventing diseases like prion-related disorders is also covered. Overall, this video provides a comprehensive overview of protein folding mechanisms.
Takeaways
- 🧬 Protein folding is a crucial biological process that makes proteins functional, and improper folding can lead to diseases like prion-related conditions.
- 🧪 The process transforms a nascent protein (linear and non-functional) into a native protein (three-dimensional and functional).
- 🧩 Protein folding can be co-translational (during protein synthesis) or post-translational (after synthesis).
- 🔬 The protein folding mechanism involves multiple stages: primary structure (linear), secondary structure (alpha helices and beta sheets), tertiary structure (3D functional), and sometimes quaternary structure (complex assemblies).
- 🌀 The primary structure is a linear sequence of amino acids with polar and non-polar side chains. This sequence determines the final folded structure.
- 💧 The secondary structure forms through hydrogen bonding interactions, resulting in alpha helices and beta sheets, which are non-functional but three-dimensional.
- 🧊 The tertiary structure forms via hydrophobic collapse, with hydrophilic regions facing outward and hydrophobic regions forming the protein core.
- 🔗 The quaternary structure, when present, involves the assembly of multiple polypeptide chains to form a fully functional protein.
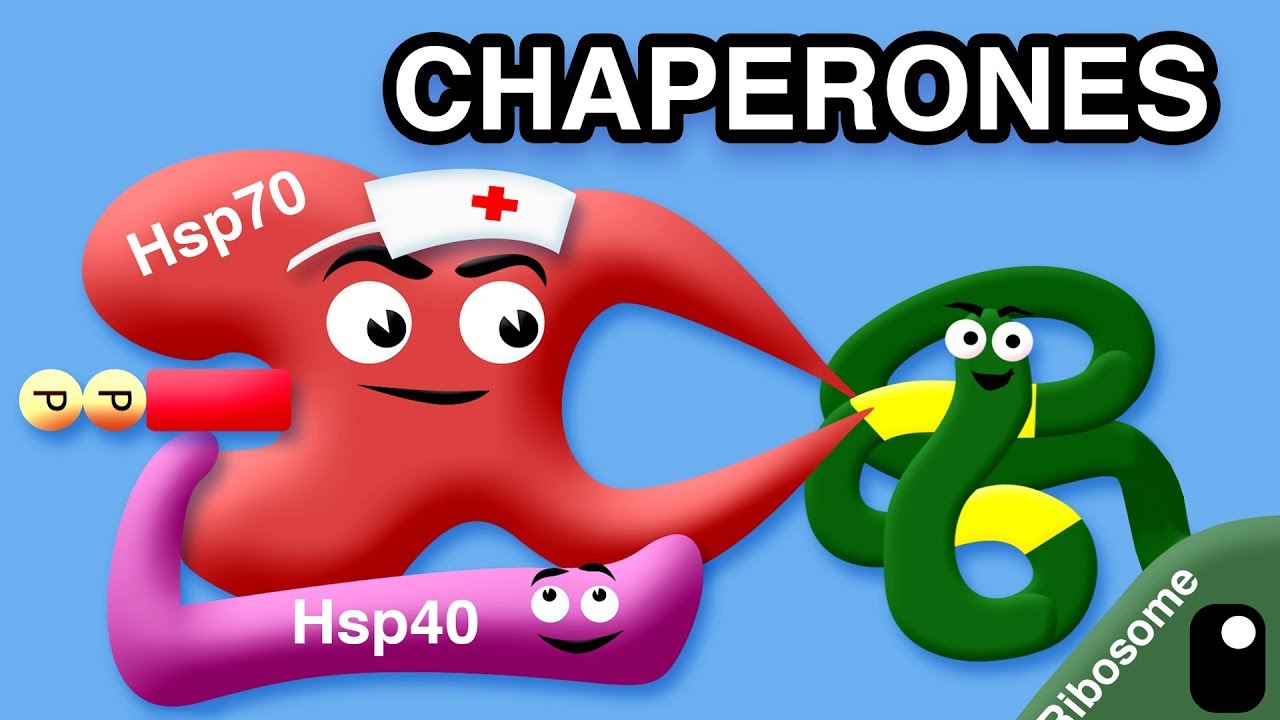
- 🛠 Chaperone proteins assist in the folding process by guiding proteins back to the correct path if they deviate from their proper folding pattern.
- 🦠 Misfolded proteins, such as prions, can resist proteases and lead to diseases like kuru, scrapie, and Alzheimer's.
Q & A
What is the significance of protein folding in biological processes?
-Protein folding is crucial because it transforms a nascent, non-functional protein into its functional, native form. Proper folding is essential for the protein to carry out its biological functions.
What can happen if protein folding does not occur correctly?
-Incorrect protein folding can render the protein non-functional and, in some cases, lead to diseases. For example, misfolded proteins can turn into prions, which can cause diseases like Kuru, Scrapie, and Alzheimer's.
What is the difference between a nascent protein and a native protein?
-A nascent protein is a newly formed protein that is arranged in a linear, one-dimensional structure and is non-functional. A native protein, on the other hand, is three-dimensional, fully folded, and functional.
What are the main stages of the protein folding process?
-Protein folding typically involves several stages: the primary structure (linear sequence of amino acids), the secondary structure (alpha helices and beta sheets), the tertiary structure (three-dimensional functional protein), and sometimes the quaternary structure (complex of multiple polypeptides).
What is co-translational protein folding?
-Co-translational protein folding occurs while the protein is still being synthesized. In this process, the N-terminus of the polypeptide chain folds while the C-terminus is still being synthesized.
How does the primary structure of a protein differ from its tertiary structure?
-The primary structure is the linear sequence of amino acids in a protein, while the tertiary structure is the three-dimensional, folded form of the protein, which is functional and stable.
What roles do alpha helices and beta sheets play in protein folding?
-Alpha helices and beta sheets are part of the protein's secondary structure, formed through hydrogen bonding. While they provide some structural stability, they are not yet fully functional forms of the protein.
What is the hydrophobic effect in protein folding?
-The hydrophobic effect refers to the tendency of hydrophobic (water-repelling) side chains in the protein to move inward, forming a hydrophobic core, while hydrophilic (water-attracting) side chains face outward, interacting with the aqueous environment. This helps stabilize the tertiary structure.
What are chaperone proteins, and how do they assist in protein folding?
-Chaperone proteins help ensure proper protein folding by guiding misfolded proteins back to the correct folding pathway. If a protein strays from its correct path, chaperones can help it refold correctly or, if that fails, degrade the misfolded protein.
What is the role of prions in diseases related to protein folding?
-Prions are misfolded proteins that become protease-resistant, allowing them to accumulate and cause neurodegenerative diseases like Kuru, Scrapie, and Alzheimer's by disrupting normal cellular functions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)