Struktur Sekunder, Tersier, Kuartener Protein | Fungsi dan Struktur Protein
Summary
TLDRThis video explains the various levels of protein structure, starting with the primary structure formed by peptide bonds linking amino acids. It then delves into secondary structures like alpha helices, beta sheets, and beta turns, all stabilized by hydrogen bonds. The tertiary structure, responsible for the three-dimensional shape of proteins, is influenced by hydrophobic interactions, disulfide bonds, hydrogen bonds, and ionic interactions. Finally, the quaternary structure involves interactions between multiple polypeptides to form a functional protein. Overall, the video provides a clear explanation of the complexity and importance of protein structures in biological functions.
Takeaways
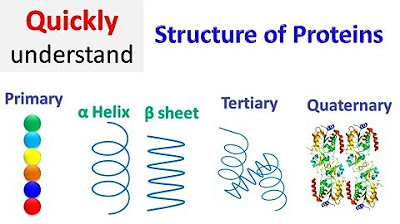
- 😀 Protein's primary structure consists of a linear chain of amino acids connected by peptide bonds, which gives it a simple, bead-like shape.
- 😀 Secondary structure involves the folding of polypeptides into common shapes such as Alpha Helix, Beta Sheet, and Beta Turn, stabilized by hydrogen bonds.
- 😀 Alpha Helix is a right-handed spiral formed by hydrogen bonds between the NH group of one amino acid and the CO group of another, creating a strong helical structure.
- 😀 Beta Sheet consists of polypeptide strands aligned in parallel or antiparallel orientations, stabilized by hydrogen bonds, forming a flat sheet structure.
- 😀 Beta Turn (also known as Beta Band or Reverse Turn) is a structural feature where the polypeptide chain bends, stabilized by hydrogen bonds between residues at the turn's ends.
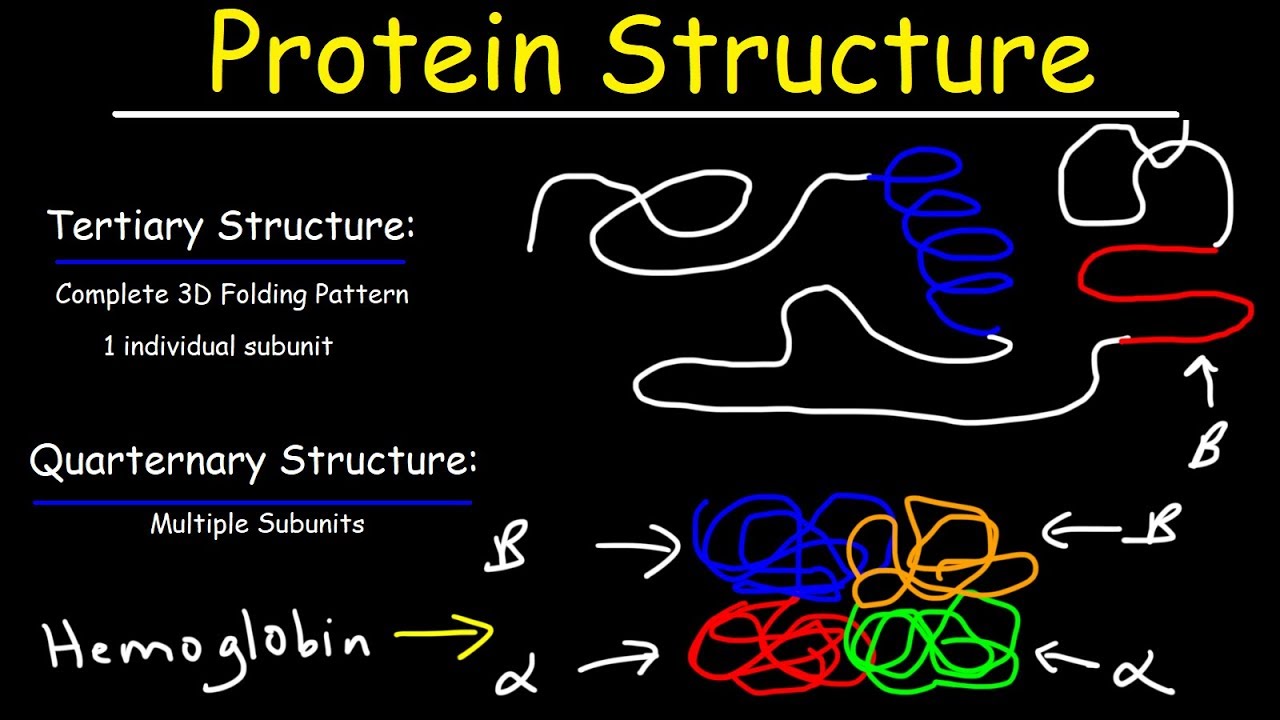
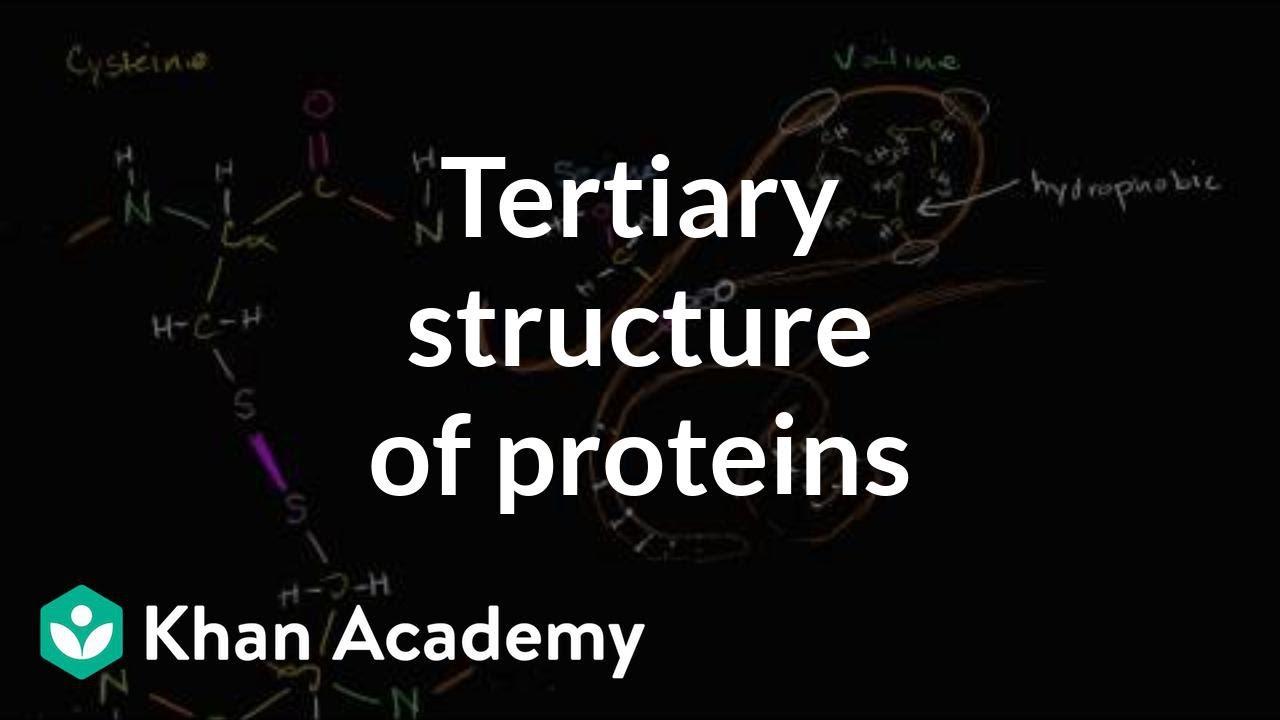
- 😀 Tertiary structure refers to the three-dimensional shape of a protein, formed through interactions between the residues in the polypeptide chain.
- 😀 The four main interactions that stabilize tertiary structure are hydrophobic interactions, disulfide bonds, hydrogen bonds, and ionic (electrostatic) interactions.
- 😀 Hydrophobic interactions occur when hydrophobic amino acids, like valine and leucine, avoid water and pack into the protein's core, while hydrophilic amino acids are on the surface.
- 😀 Disulfide bonds are covalent bonds formed between cysteine residues and help stabilize the protein structure, especially in extracellular environments.
- 😀 Quaternary structure refers to the interaction between multiple polypeptide chains (subunits) to form a functional protein, typically stabilized by non-covalent interactions.
Q & A
What is the primary structure of a protein?
-The primary structure of a protein is the sequence of amino acids linked together by peptide bonds. This linear chain forms the foundation for higher levels of protein structure.
How does a polypeptide chain adopt its secondary structure?
-The secondary structure of a protein is formed when the polypeptide chain, through interactions between the amino acids, adopts regular shapes like alpha helices and beta sheets. These are stabilized by hydrogen bonds between the amino acid residues.
What is the difference between alpha helices and beta sheets in secondary protein structure?
-Alpha helices are spiral shapes where the polypeptide chain coils around a central axis, while beta sheets consist of parallel or antiparallel arrangements of polypeptide strands. Both are stabilized by hydrogen bonds between the residues.
What stabilizes the alpha helix structure of a protein?
-The alpha helix structure is stabilized by hydrogen bonds between the NH group of one amino acid and the CO group of another, with every 3.6 residues participating in each turn of the helix.
What are beta turns, and how do they relate to protein structure?
-Beta turns, or reverse turns, are structures that allow the polypeptide chain to change direction. These are important for connecting different elements of secondary structure, like alpha helices and beta sheets.
What factors contribute to the tertiary structure of a protein?
-The tertiary structure of a protein is determined by the interactions between the side chains of amino acids within the polypeptide. These include hydrophobic interactions, disulfide bonds, hydrogen bonds, and ionic bonds.
How do hydrophobic interactions affect protein structure?
-Hydrophobic interactions cause hydrophobic amino acids to cluster in the interior of the protein, away from the aqueous environment, which helps stabilize the protein's three-dimensional structure.
What is the role of disulfide bonds in protein structure?
-Disulfide bonds are covalent bonds between the sulfur atoms of cysteine residues. They stabilize the tertiary structure of a protein, especially in extracellular environments, by preventing denaturation.
What is the quaternary structure of a protein?
-The quaternary structure of a protein refers to the interaction between multiple polypeptide chains or subunits. These subunits interact non-covalently to form a functional protein complex.
How do proteins with a quaternary structure differ from those with only a tertiary structure?
-Proteins with a quaternary structure consist of multiple polypeptide subunits interacting with each other, whereas proteins with only a tertiary structure are made up of a single polypeptide chain folded into its three-dimensional shape.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)