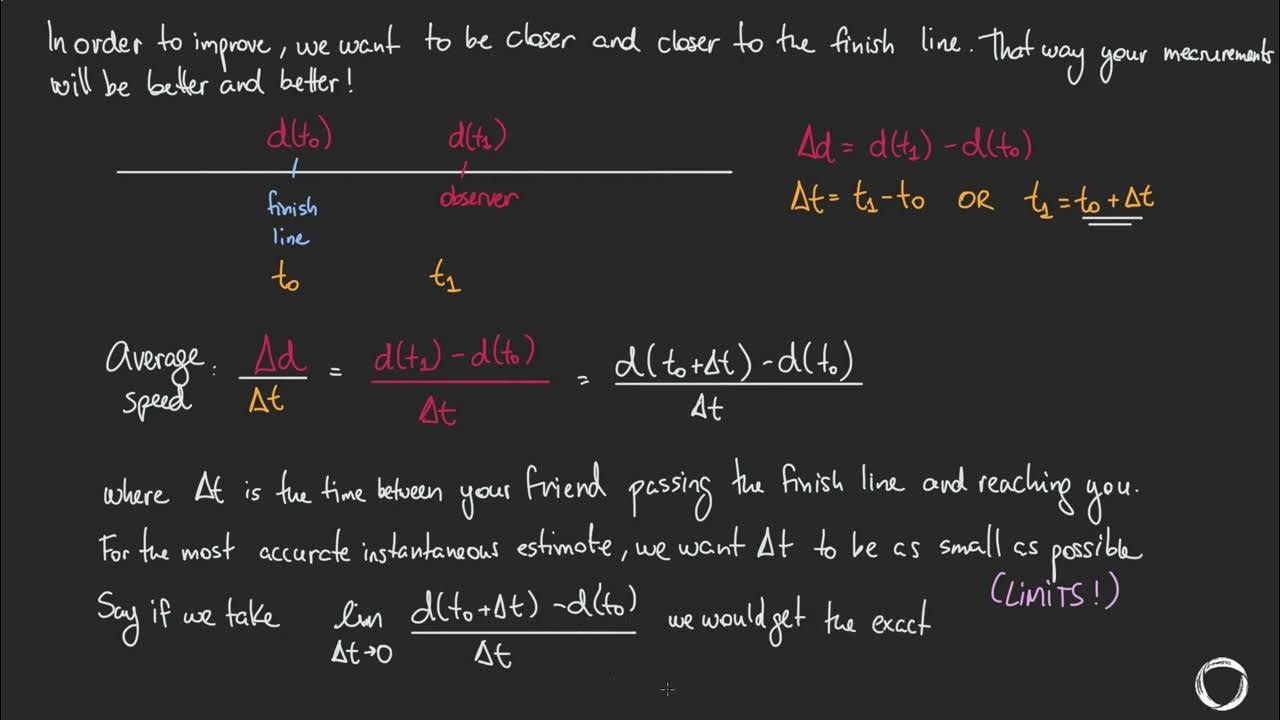GCSE Chemistry - How to Calculate the Rate of Reaction - Measuring Rate of Reaction #48
Summary
TLDRThis educational video explains how to use graphs to measure the mean and instantaneous rates of a chemical reaction. It demonstrates calculating average reaction rates over a period and determining rates at specific times by analyzing the gradient of the reaction curve. The example of magnesium and hydrochloric acid reaction is used to illustrate these concepts, showing how the rate changes from fast at the beginning to slow as the reaction progresses. The video reassures viewers that approximate tangent lines are acceptable for calculating reaction rates.
Takeaways
- 📊 The video explains how to use graphs to measure the mean rate of a chemical reaction and the rate at a specific time.
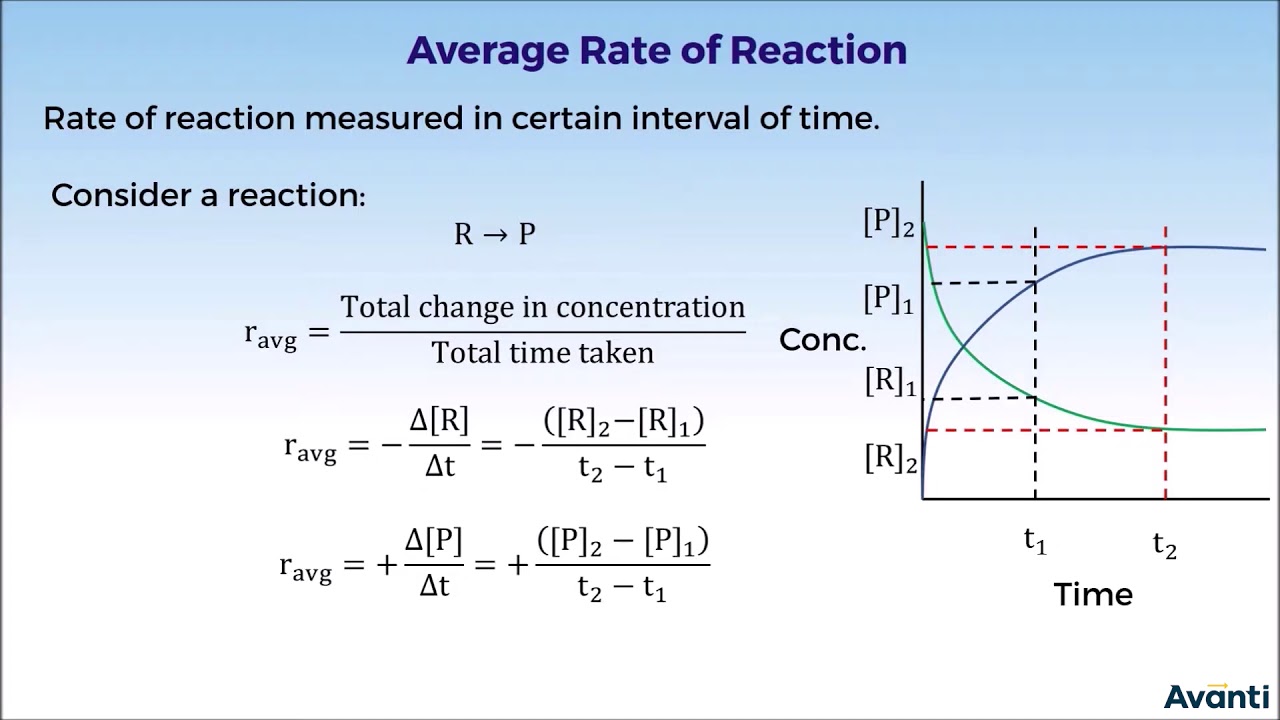
- ⏱️ The rate of reaction is calculated by dividing the amount of reactants used or products formed by the time taken for the change.
- 🔍 A graph with time on the x-axis and volume of hydrogen on the y-axis can illustrate how the rate of reaction changes over time.
- ➡️ Initially, the reaction rate is high, indicated by a steep curve, and slows down as reactants are consumed, eventually plateauing.
- 📉 To find the mean rate over a period, calculate the volume of hydrogen produced during that time and divide by the time in seconds.
- 📈 The gradient of the curve at a specific time point represents the instantaneous rate of reaction at that time.
- 📐 To calculate the rate at a specific time, draw a tangent to the curve at the corresponding x-axis value and find its gradient.
- 🔍 The gradient is found by the change in volume (y-axis) divided by the change in time (x-axis), with the tangent line extended to intersect the axes.
- 🔬 The video also mentions that the same method can be applied to a graph plotting the amount of magnesium remaining against time.
- ⚖️ For a magnesium graph, the rate of reaction is calculated by the change in magnesium weight divided by the change in time, reflecting the reaction's progress.
Q & A
How is the rate of reaction calculated in the context of the video?
-The rate of reaction is calculated by dividing the amount of reactants used or the amount of product formed over the time taken for that change to occur.
What is the difference between the average rate and the instantaneous rate of a reaction?
-The average rate is calculated over a period of time, giving a general idea of how fast the reaction is proceeding, while the instantaneous rate is the rate at a specific moment in time, which can be determined by the slope of the curve on a graph at that point.
Why does the rate of reaction typically slow down as the reaction progresses?
-The rate of reaction slows down because as the reaction progresses, the concentration of reactants decreases, leading to fewer effective collisions between reactant particles.
How can you determine the mean rate of reaction over a specific period using a graph?
-To determine the mean rate of reaction over a specific period, you find the volume of product formed during that period on the graph, and then divide that volume by the time interval.
What does a steep curve on a graph of reaction rate versus time indicate?
-A steep curve on a graph of reaction rate versus time indicates a high rate of reaction, suggesting that a large amount of product is being formed in a short period of time.
How do you calculate the rate of reaction at a specific time using a graph?
-To calculate the rate of reaction at a specific time, you draw a tangent to the curve at the corresponding time on the x-axis, then determine the gradient of this tangent, which is the change in volume of product divided by the change in time.
Why is it important to make the tangent as long as possible when calculating the instantaneous rate?
-Making the tangent as long as possible helps to minimize the error in estimating the gradient, as it allows for a more accurate measurement of the change in y and x values.
What is the significance of a graph plateauing in the context of a reaction rate graph?
-A graph plateauing indicates that the reaction has completed, and no more product is being formed, suggesting that all the reactants have been consumed.
Can the method described in the video be applied to reactants as well as products?
-Yes, the method can be applied to reactants as well. You can plot the amount of a reactant remaining against time and use a similar approach to determine the rate of reaction.
How does the examiner's tolerance for the accuracy of tangents affect the calculation of the rate of reaction?
-The examiner's tolerance for the accuracy of tangents allows for a range of values in the calculation, meaning that while it's important to be careful, slight variations in the tangent's placement won't significantly affect the final rate calculation.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)