Lewis Dot Structures
Summary
TLDRProfessor Dave's tutorial on Lewis dot structures enlightens viewers on the representation of atoms and their valence electrons, crucial for understanding covalent bonding. The video explains how to depict atoms, their coordination sites, and the formation of covalent bonds, including single, double, and triple bonds. It also touches on formal charges and the tendency of atoms like carbon, nitrogen, oxygen, and fluorine to form specific numbers of bonds. The tutorial is designed to enhance comprehension of molecular structures and chemical bonding.
Takeaways
- 🔬 Lewis dot structures represent atoms and their valence electrons in a chemical context.
- 🌐 Atoms are depicted by their chemical symbols, with valence electrons shown as dots around them.
- 📊 Carbon, for example, has four valence electrons and is represented with four dots in its outermost shell.
- 🔄 The drawing of valence electrons follows a specific order: place one electron per coordination site before pairing them up.
- 👫 Elements in the same group have similar Lewis dot symbols due to the same number of valence electrons.
- 🤝 In Lewis dot structures for molecules, unpaired electrons from different atoms can form covalent bonds, represented as lines.
- 🔗 The number of valence electrons an atom has influences how many bonds it forms, such as carbon forming four bonds.
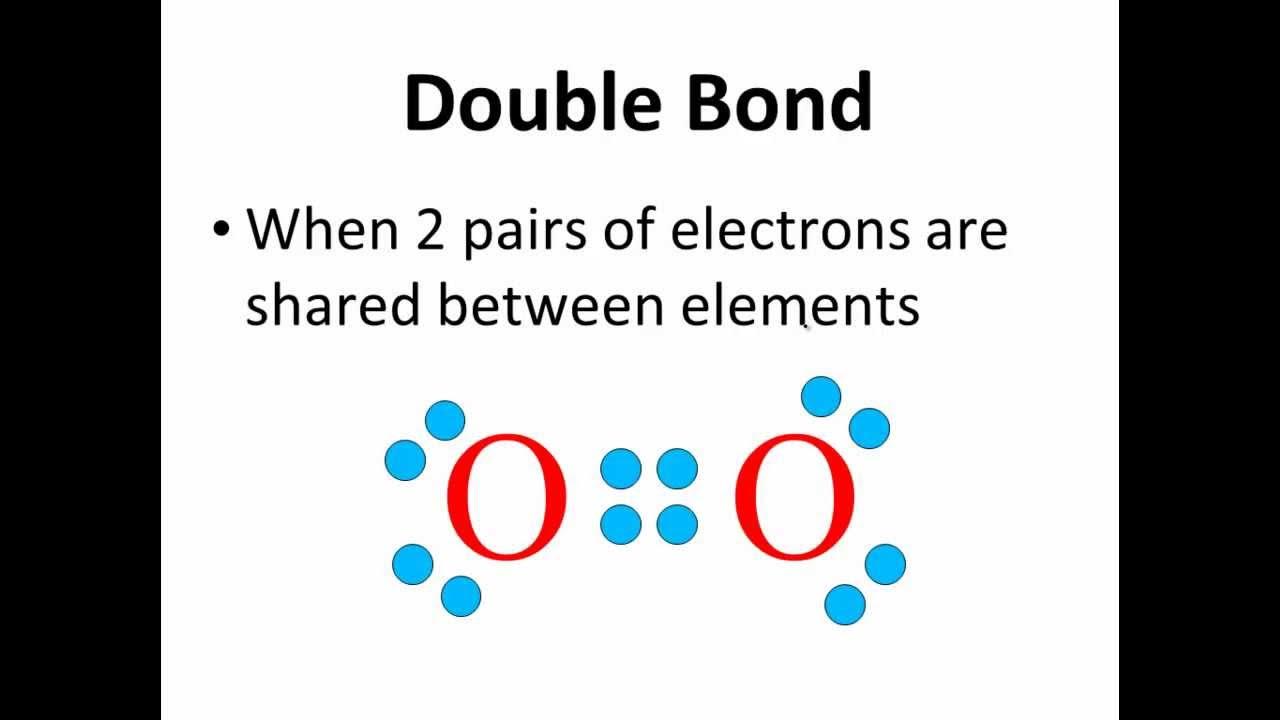
- 💠 Atoms can form single, double, or triple bonds, with the latter being the shortest in length.
- 🎯 Formal charges may appear in Lewis dot structures when an atom contributes a different number of electrons than its typical valence.
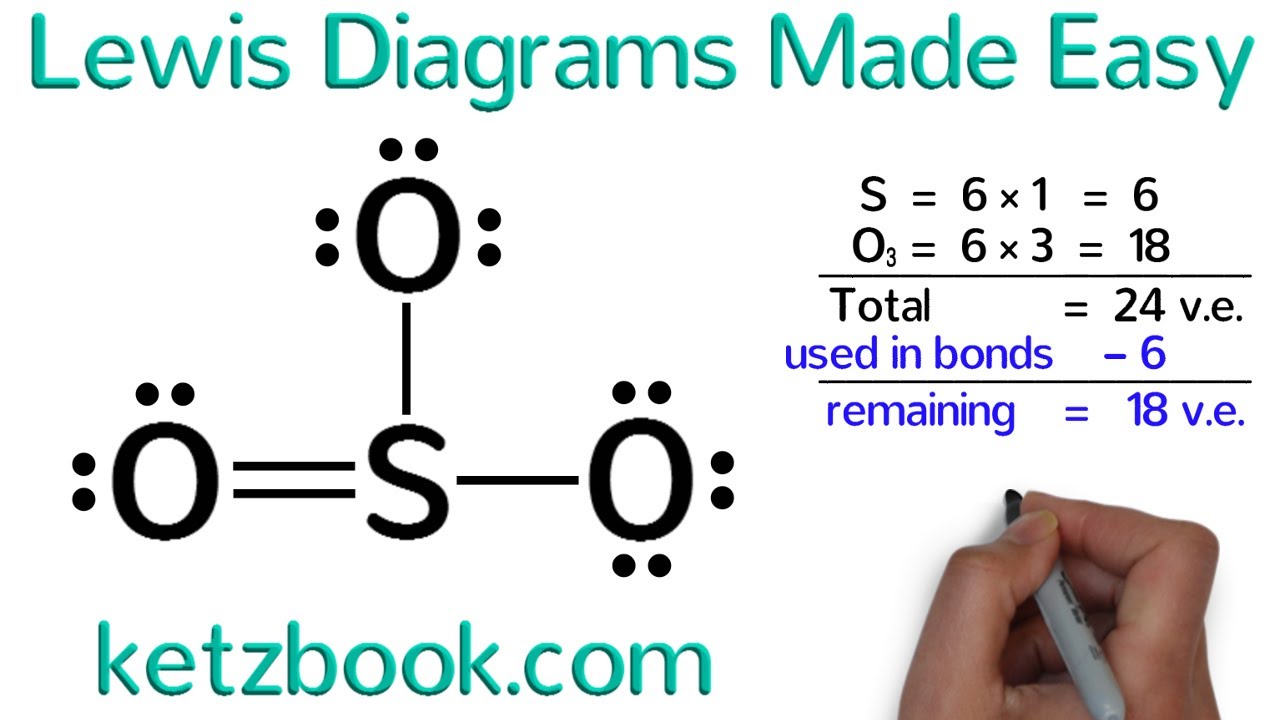
- 🌀 Larger atoms like phosphorus and sulfur can form more than four covalent bonds, aiming to fill their outermost shell with eight electrons.
Q & A
What is the purpose of learning how to draw Lewis dot structures?
-The purpose of learning how to draw Lewis dot structures is to visually represent the valence electrons of atoms and how they form covalent bonds with other atoms, which is crucial for understanding the structure of molecules.
How are valence electrons represented in a Lewis dot structure?
-Valence electrons are represented as dots around the chemical symbol of an atom. For example, carbon, which has four valence electrons, is represented with four dots around the 'C' symbol.
Why do we assume there are four coordination sites for small atoms like carbon?
-We assume there are four coordination sites for small atoms like carbon because that is the number needed for such atoms to fill their outermost shell, or octet, by forming covalent bonds.
How do you draw the valence electrons for an atom like carbon?
-For carbon, you first place one dot in each coordination site before pairing them up, resulting in a structure that looks like this: C:..., not like this: C::.
What is a covalent bond in the context of Lewis dot structures?
-A covalent bond in Lewis dot structures is represented by a line between two atoms, indicating that unpaired electrons from each atom have combined to form a bond, which always contains two electrons.
What is a sigma bond and how does it differ from a pi bond?
-A sigma bond is the first covalent bond formed between two atoms. A pi bond is the second bond in a double bond or the second and third bonds in a triple bond. Pi bonds are represented in Lewis structures as lines that form a 'sideways' bond.
Why do single, double, and triple bonds differ in length?
-Single covalent bonds are the longest because they consist of one pair of electrons between the atoms. Double bonds are shorter because they have two pairs of electrons closer together, and triple bonds are the shortest due to three pairs of electrons being even closer.
What is a formal charge and how does it relate to Lewis dot structures?
-A formal charge is the charge an atom appears to have in a Lewis dot structure when the number of electrons it is contributing differs from its typical valence. It is calculated by the formula: Formal charge = (Valence electrons of the atom) - (Non-bonding electrons) - (1/2 × Bonding electrons).
Why does nitrogen in ammonia have a neutral charge while in the ammonium ion it has a formal positive charge?
-In ammonia (NH3), nitrogen contributes all five of its valence electrons, one per covalent bond, and has no formal charge. In the ammonium ion (NH4+), nitrogen contributes four electrons to the structure, one less than its typical valence, resulting in a formal positive charge.
How do larger atoms like phosphorus and sulfur form more than four covalent bonds?
-Larger atoms like phosphorus and sulfur can have more than four valence electrons and thus can form more than four covalent bonds. They can expand their octet by utilizing d-orbitals to accommodate more than eight electrons, a phenomenon known as 'expanded octet.'
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)