Fluorine ***
Summary
TLDRThis guide provides essential information about the chemical element fluorine, aimed at chemistry students. It explains fluorine's chemical symbol (F), atomic number (9), and historical background, including its discovery and isolation. The guide also details fluorine's physical and chemical properties, such as its pale yellow color, high reactivity, and toxicity. Additionally, it discusses fluorine's classification as a halogen and its various uses, from preventing tooth decay in water supplies to applications in air conditioning, refrigeration, and as an insecticide.
Takeaways
- 🔤 The chemical symbol for fluorine is 'F', derived from its mineral source 'fluorite'.
- 🔢 Fluorine has an atomic number of 9, which corresponds to the number of protons in its nucleus.
- 📚 The name 'fluorine' was coined by Sir Humphrey Davy in 1813, inspired by the mineral 'fluorite'.
- 🔬 First described in 1529, fluorine was successfully isolated by Henry Moissan in 1886 through electrolysis.
- 🏆 Moissan was awarded the Nobel Prize in Chemistry in 1906 for his work in isolating fluorine.
- 🌐 Fluorine is a pale yellow-white or colorless gas, sometimes exhibiting fluorescence.
- ⚗️ It is highly reactive, combining with all elements except helium, neon, and argon.
- 💧 When mixed with water, fluorine reacts explosively and is highly corrosive.
- 🦷 Fluorine's compounds are used in water treatment to prevent tooth decay and are found in toothpaste.
- 🛠️ Beyond dental applications, fluorine is used in the extraction of uranium, air conditioning, refrigeration, and as an insecticide.
- 📊 The properties of fluorine, including melting and boiling points, atomic mass, and density, contribute to its various industrial uses.
Q & A
What is the chemical symbol for fluorine?
-The chemical symbol for fluorine is 'F'.
Who coined the name 'fluorine' and when?
-The name 'fluorine' was coined by Sir Humphry Davy in 1813.
What is the atomic number of fluorine, and what does it represent?
-The atomic number of fluorine is 9, which represents the number of protons in the nucleus of a fluorine atom.
What are some physical properties of fluorine?
-Fluorine is a pale yellow, white, or colorless gas that can sometimes be fluorescent. It has a pungent smell and is 1.3 times as dense as air.
How was fluorine first successfully isolated, and by whom?
-Fluorine was first successfully isolated by French chemist Henri Moissan in 1886 through the electrolysis of potassium fluoride and hydrochloric acid.
What is the significance of fluorine in the group called halogens?
-Fluorine is part of the halogen group, which consists of five elements located in group 17 of the periodic table. Halogens are highly reactive non-metallic elements with low melting and boiling points.
What are the common uses of fluorine?
-Fluorine is used in toothpaste, in the extraction of uranium, in air conditioning and refrigeration, as an insecticide, and in the production of fluorocarbons like Teflon, which are used as lubricants.
What are some chemical properties of fluorine?
-Fluorine is highly toxic, corrosive, and flammable. It reacts with nearly all elements except helium, neon, and argon, and it reacts explosively with water.
What are fluorides, and why are they important?
-Fluorides are compounds of fluorine, and they are important because they are added to public water supplies to reduce the incidence of tooth decay.
What is the historical significance of the mineral 'fluorite' in the context of fluorine?
-The mineral 'fluorite' is the chief ore of fluorine, and it was named by Georgius Agricola in 1546. Fluorine's name is derived from 'fluor,' which means 'flow' in Latin, reflecting its use as a flux in metallurgy.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

10 topics=150+ Marks in CSIR NET Chemistry| Most Important Topics for CSIR NET Chemical Science 2024

REAKSI REDOKS DAN ELEKTROKIMIA - MATERI KIMIA KELAS 12 | Edcent.id

Penyetaraan Persamaan Reaksi Kimia dengan Cepat- Kimia Kelas 10

UNSUR SENYAWA DAN CAMPURAN | KLASIFIKASI MATERI

2nd Year Chemistry | IPE | Best Strategy | IPE 2023 | Ramadevi Mam | Vedantu Telugu
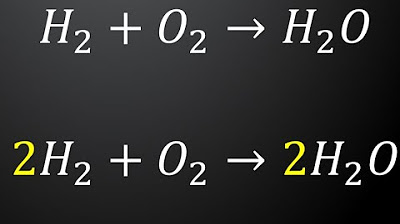
Chemistry: Balancing Chemical Equations (Tagalog Explained)
5.0 / 5 (0 votes)