Cálculo do Fator de Empacotamento CFC e CS - Exercícios Resolvidos Callister (07)
Summary
TLDRThis educational video script focuses on exercises related to the calculation of the unit cell volume and packing factor of simple cubic and face-centered cubic crystal structures. The instructor guides through determining the unit cell volume in relation to atomic radius and explains the concept of packing factor by comparing the volume occupied by atoms to the cell volume. The script includes visual aids and 3D models to clarify the number of atoms within the unit cells and their arrangement, ultimately revealing the packing efficiency of these crystalline structures.
Takeaways
- 🔬 The lecture focuses on exercises related to the calculation of the unit cell volume and packing factor of simple cubic and face-centered cubic structures.
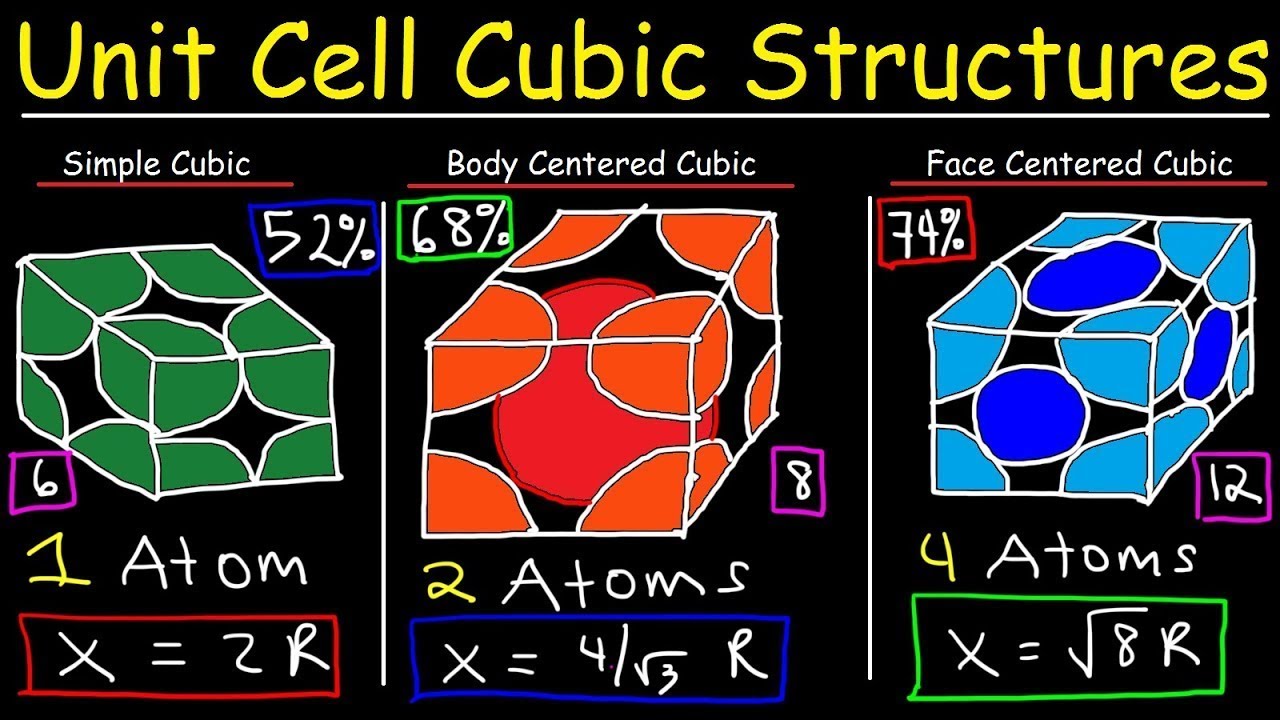
- 📐 In a simple cubic structure, the unit cell volume is calculated to be 8R^3, where R is the atomic radius.
- 📊 The packing factor for a simple cubic structure is approximately 0.52, indicating a lower density of atomic packing.
- 🔍 For a face-centered cubic structure, the unit cell volume is determined to be 16R^3, with R being the atomic radius.
- 🔢 The packing factor in a face-centered cubic structure is calculated to be around 0.74, which is higher than that of a simple cubic structure, indicating a denser atomic arrangement.
- 🧠 The number of atoms in a simple cubic unit cell is one, while in a face-centered cubic unit cell, it's calculated to be four.
- 📚 The lecture emphasizes the importance of understanding the theoretical part of the material before attempting exercises for better comprehension.
- 📖 The script mentions that the theoretical part of the lecture is available in a separate video, which is recommended for viewing to aid in understanding the exercises.
- 💡 The exercises are designed to help students understand the relationship between atomic radius, unit cell volume, and packing factor in different crystal structures.
- 👨🏫 The instructor uses visual aids and 3D models to explain the atomic arrangement within the unit cells and how it affects the packing factor.
- 🤔 The lecture concludes with an invitation for students to ask questions if they have any doubts, encouraging interaction and clarification.
Q & A
What is the main topic of the video?
-The main topic of the video is the calculation of the unit cell volume and the packing factor of simple cubic and face-centered cubic structures in materials science.
What is the relationship between the edge of a simple cubic cell and the atomic radius?
-The edge of a simple cubic cell is twice the atomic radius, as each edge of the cube is defined by the distance between two atomic centers.
How is the unit cell volume of a simple cubic structure calculated?
-The unit cell volume of a simple cubic structure is calculated by cubing the edge length, which is 2R where R is the atomic radius, resulting in 8R^3.
What is the packing factor of a simple cubic structure?
-The packing factor of a simple cubic structure is calculated by dividing the volume occupied by the atoms (using the rigid sphere model) by the unit cell volume. For a simple cubic structure, it is (4/3)πR^3/8R^3 = π/6 ≈ 0.52.
What is the difference between a simple cubic and a face-centered cubic structure?
-In a simple cubic structure, each corner of the cube contains one atom, while in a face-centered cubic structure, atoms are also located at the center of each face of the cube.
How is the unit cell volume of a face-centered cubic structure related to the atomic radius?
-The unit cell volume of a face-centered cubic structure is related to the atomic radius by the formula V = (16/3)a^3, where a is the edge length of the cube, and a = 2R for the face-centered cubic structure.
What is the number of atoms per unit cell in a face-centered cubic structure?
-In a face-centered cubic structure, there are 4 atoms per unit cell, with one atom at each corner and one atom at the center of each face of the cube.
How is the packing factor calculated for a face-centered cubic structure?
-The packing factor for a face-centered cubic structure is calculated by dividing the total volume of the atoms (considering each as a sphere of radius R) by the unit cell volume, which results in a packing factor of approximately 0.74.
Why is the packing factor lower in a simple cubic structure compared to a face-centered cubic structure?
-The packing factor is lower in a simple cubic structure because the atoms are less efficiently packed, with more empty space between them compared to the face-centered cubic structure where atoms are located at the centers of the cube faces, leading to a denser packing.
What does the video suggest about the compactness of metals with a simple cubic structure?
-The video suggests that metals with a simple cubic structure are not very compact, as indicated by the low packing factor, which is not typical for metals where a more efficient packing is usually observed.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

The Structure of Crystalline Solids

5 - Class 12 - Chemistry - Solid State - Calculation of number of atoms per unit cell

Aula 10 – Estruturas Cristalinas Cúbicas de Face Centrada, Corpo Centrado e Hexagonal Compacta.
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu

AMIE Exam Lectures- Materials Science & Engineering | BCC | FCC | HCP | Cubic System | 3.2

Lattice Structures Part 1
5.0 / 5 (0 votes)