noc19-cy16 Lecture 06-Solid state Chemistry-Week 2 Lecture-1 Unit Cell
Summary
TLDRThis lecture delves into the concept of unit cells in crystallography, highlighting their importance in defining the ordered structure of crystals. It discusses the translational symmetry of crystals and how unit cells, whether primitive or conventional, can be replicated to construct the entire crystal lattice. The instructor introduces various unit cell shapes, including cubic, tetragonal, and triclinic, emphasizing the restrictions on their dimensions and angles due to space-filling requirements. The talk also touches on visualization tools like VESTA for better understanding, and sets the stage for further exploration of crystal systems and lattices.
Takeaways
- 📐 The script introduces the concept of Unit Cells, explaining that they are the repeating units of a crystal, essential for generating the entire crystal structure through translation.
- 🔍 The importance of translational symmetry in crystals is highlighted, showing that by repeatedly translating the unit cell, one can construct the entire crystal without voids.
- 🛠️ Visualization tools like VESTA software are recommended for better understanding of crystalline solids, emphasizing the need to go beyond 2D representations and explore 3D models.
- 📏 The script discusses different shapes of unit cells, including primitive and conventional unit cells, and how they can vary in size and orientation within a crystal lattice.
- 📊 Unit cells must fill the entire space without leaving voids when translated, which restricts the possible shapes they can take, such as not allowing right-angled triangles as unit cells in 2D.
- 🔄 The concept of parallelepiped is introduced as a general shape for a unit cell in 3D, defined by three vectors (a, b, c) and three angles (alpha, beta, gamma).
- 📉 The script outlines various restrictions on unit cell dimensions and angles, leading to different types of unit cells such as triclinic, monoclinic, orthorhombic, tetragonal, trigonal, and cubic.
- 🔑 The significance of symmetry in unit cells is discussed, with the unit cell chosen to reflect the same point symmetry as the crystal itself, including rotations, reflections, and inversions.
- 🌐 The script touches on the concept of crystal systems, which categorize crystals into seven distinct systems based on unit cell symmetry and shape.
- 📚 The importance of understanding both primitive and conventional unit cells is mentioned, as they provide different perspectives on the crystal structure and its properties.
- 🔬 The lecture concludes with a teaser for the next class, where the discussion will delve deeper into unit cells, lattices, and the broader topic of crystallography.
Q & A
What is the main topic of discussion for week 2 of the course?
-The main topic of discussion for week 2 is Unit Cells, including Primitive and Conventional Unit Cells, as well as lattices, Bravais Lattices, and the concept of a crystal.
What characterizes a crystalline solid?
-A crystalline solid is characterized by an ordered arrangement of its constituent units, with the repeating unit of a crystal being referred to as a unit cell.
How can the entire crystal be generated from the unit cell?
-The entire crystal can be generated from the unit cell by repeatedly translating the unit cell in all directions, which is possible due to the translational symmetry of crystals.
What is the significance of the software package VESTA in the context of this course?
-VESTA is a well-known, freely available software package that helps visualize crystal structures, which is recommended for students to enhance their understanding of crystalline solids.
What is the difference between a Primitive Unit Cell and a Conventional Unit Cell?
-The script does not provide a direct explanation of the difference between Primitive and Conventional Unit Cells, but it is implied that different choices of unit cells exist, which will be discussed in more detail in the course.
Why is it important to consider two-dimensional representations when discussing unit cells?
-Two-dimensional representations are used because they are easier to draw and visualize, allowing for a better understanding of the concepts, although the actual crystals are three-dimensional.
What is the relationship between the shape of the unit cell and the symmetry of the crystal?
-The unit cell is chosen to have the same point symmetry as the crystal itself, which includes rotations, reflections, and inversions.
What are the common shapes of unit cells in 3D?
-The common shapes of unit cells in 3D are cubic, tetragonal, orthorhombic, triclinic, monoclinic, trigonal, and hexagonal.
What is a parallelepiped and how is it related to the unit cell?
-A parallelepiped is a three-dimensional solid object that can fill space. It is related to the unit cell as the general shape of a unit cell can be described by a parallelepiped with vectors a, b, and c, and angles alpha, beta, and gamma.
What are the restrictions on the angles and lengths of the vectors in a unit cell?
-The restrictions on the angles and lengths of the vectors in a unit cell are related to the symmetry of the crystal. For example, in a cubic unit cell, all angles are 90 degrees and all lengths are equal, whereas in a triclinic unit cell, there are no restrictions on the angles or lengths.
What are the 7 crystal systems and how do they relate to the unit cell shapes?
-The 7 crystal systems are triclinic, monoclinic, orthorhombic, tetragonal, trigonal (hexagonal is a special case of trigonal), and cubic. These systems classify all possible crystal structures based on the symmetry of their unit cells.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Lecture 3 Part 1 - Crystal Structure - 2 (Unit Cell, Lattice, Crystal)
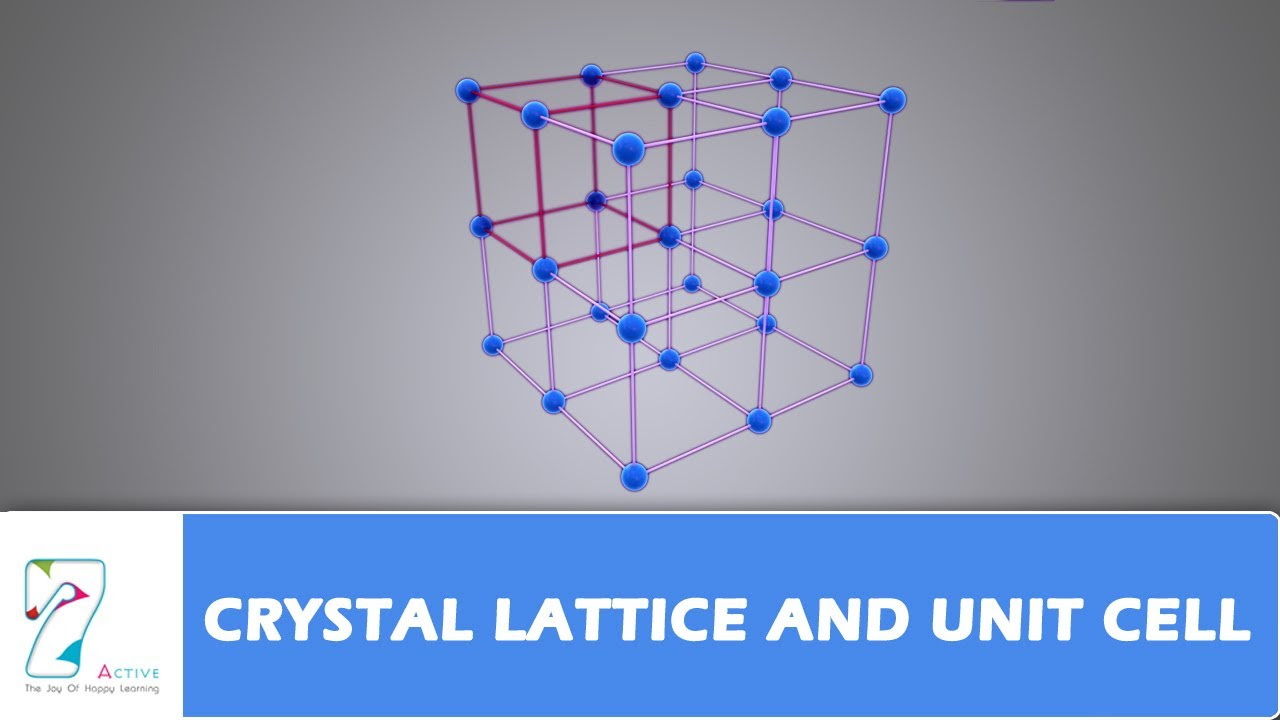
CRYSTAL LATTICE AND UNIT CELL

(1 dari 3) Farmasi Fisika 2021 - Kristal - Pendahuluan, Kristalisasi, Kristalografi & Jenis Kristal

Aula 10 – Estruturas Cristalinas Cúbicas de Face Centrada, Corpo Centrado e Hexagonal Compacta.

1. Cell & Subcellular Organelles - BASIC STRUCTURE OF THE CELL

What is a Unit Cell?
5.0 / 5 (0 votes)