4.CHEMICAL THERMODYNAMICS| EASY TRICK TO LEARN|ONE SHOT | COMPLETE CHAPTER IN 20 MINS|PRADEEP SIR
Summary
TLDRThis video lesson offers a comprehensive overview of key thermodynamic concepts, including ionization, atomization, Hess's Law, and Gibbs Free Energy. It explores enthalpy changes in chemical reactions, standard states, and the principles of entropy and spontaneity in processes. The speaker highlights the importance of understanding the relationship between enthalpy, entropy, and temperature in determining the spontaneity of reactions. By breaking down these fundamental concepts in a clear and engaging manner, the video aims to make complex thermodynamic principles easily accessible for students, preparing them for practical application in solving problems.
Takeaways
- 😀 Ionization involves removing an electron from a mole of gaseous atoms, whereas atomization refers to dissociating a mole of a gaseous substance.
- 😀 The thermodynamic standard state is defined as the stable state of a substance at 298 K and 1 bar pressure.
- 😀 Enthalpy of a chemical reaction is the difference between the sum of the enthalpy of the products and reactants.
- 😀 Combustion enthalpy is the enthalpy change for the complete combustion of one mole of a substance in its standard state.
- 😀 Bond enthalpy refers to the energy required to break one mole of bonds in gaseous molecules.
- 😀 Hess’s Law states that the overall enthalpy change for a reaction is the sum of the individual enthalpy changes of the steps involved.
- 😀 Entropy is the ratio of heat transfer to temperature and is a state function, measured in joules per kelvin.
- 😀 The second law of thermodynamics states that in a spontaneous process, the total entropy of the system increases.
- 😀 Gibbs Free Energy (ΔG) helps determine the spontaneity of a process; a negative value indicates a spontaneous process.
- 😀 Standard conditions for thermodynamic calculations are 298 K temperature and 1 bar pressure, referred to as the standard state.
- 😀 Quick revision and repeated learning through video lessons can help students grasp thermodynamic concepts effectively.
Q & A
What is ionization in thermodynamics?
-Ionization refers to the process of removing one electron from a mole of gaseous atoms, transforming them into ions.
What does atomization mean in thermodynamic terms?
-Atomization is the process of dissociating a mole of a gaseous substance into its individual atoms.
What is the thermodynamic standard state?
-The thermodynamic standard state refers to the stable state of a substance at 298 K (25°C) and 1 bar pressure. This state serves as a reference for thermodynamic calculations.
How is the enthalpy of a chemical reaction defined?
-The enthalpy of a chemical reaction is the difference between the sum of the enthalpies of the products and the sum of the enthalpies of the reactants.
What is the enthalpy of combustion?
-The enthalpy of combustion is the energy change that occurs when one mole of a substance undergoes complete combustion in its standard state.
What does bond enthalpy refer to?
-Bond enthalpy refers to the energy required to break one mole of a particular covalent bond in a gaseous molecule, resulting in the formation of gaseous atoms and radicals.
What is Hess's Law and how does it relate to enthalpy?
-Hess's Law states that the overall enthalpy change of a reaction is equal to the sum of the enthalpy changes of the individual steps involved in the reaction.
What is entropy in thermodynamics?
-Entropy is a measure of disorder or randomness in a system. It is defined as the ratio of heat transfer to temperature, and is a state function.
How does the Second Law of Thermodynamics relate to entropy?
-The Second Law of Thermodynamics states that the total entropy of an isolated system can never decrease, and it dictates the spontaneity of processes. Spontaneous processes increase the total entropy.
What is Gibbs Free Energy and how does it determine spontaneity?
-Gibbs Free Energy (ΔG) is the energy available to do work in a system. If ΔG is negative, the process is spontaneous. The relationship is given by ΔG = ΔH - TΔS.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Hukum Termodinamika, Bagian 1: Energi Dalam dan Hukum Pertama

Thermodynamics Class 11 in 5 Minutes | Chemistry | Quick Revision | NEET, JEE & CBSE |

Introduction of Bioenergetics Part 1

Clase nº 3 - 1. Equilibrio quimico: el avance de reacción y la constante de equilibrio
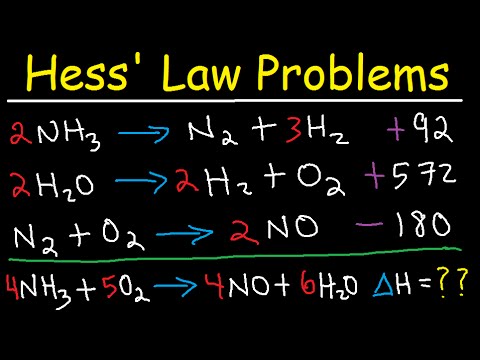
Hess Law Chemistry Problems - Enthalpy Change - Constant Heat of Summation

Fisika Kelas 11 | Termodinamika
5.0 / 5 (0 votes)
