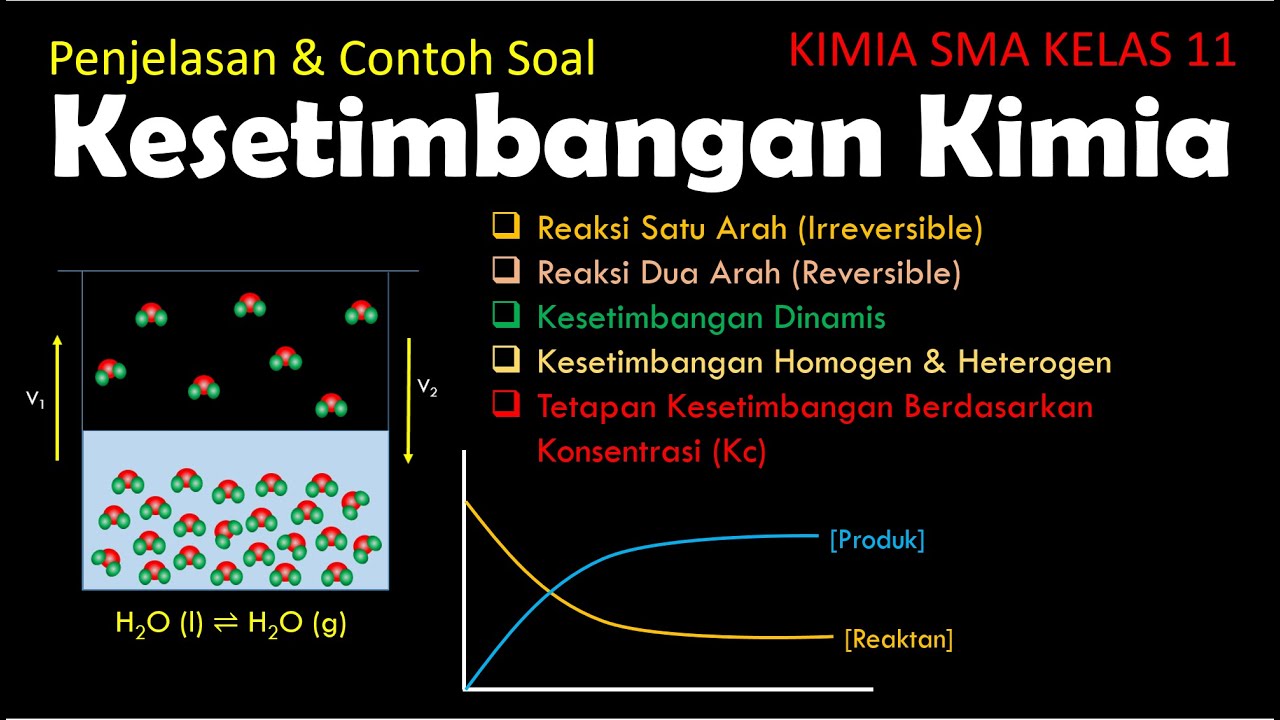
Clase nº 3 - 1. Equilibrio quimico: el avance de reacción y la constante de equilibrio
Summary
TLDRThis video delves into the concept of chemical equilibrium, explaining the key thermodynamic analysis used to understand multi-component systems at equilibrium. It defines chemical equilibrium as the state where the molar fractions of each component remain constant over time, and highlights the importance of the Gibbs free energy in determining spontaneity and equilibrium. The video explores reaction progress, the concept of reaction advancement, and how temperature and entropy influence the system's behavior. It concludes by relating the equilibrium constant to the Gibbs free energy and discusses how reaction direction and coefficients affect equilibrium values.
Takeaways
- 😀 Chemical equilibrium occurs when the molar fraction of each component remains constant over time, indicating no net change in the system's composition.
- 😀 A system in equilibrium involves both forward and reverse reactions occurring at the same rate, resulting in a stable composition.
- 😀 Thermodynamic analysis, particularly Gibbs free energy, is crucial in determining whether a chemical process will be spontaneous.
- 😀 The Gibbs free energy decreases as a reaction progresses, with equilibrium reached when ΔG equals zero.
- 😀 Reaction progress is measured by the reaction advancement variable (ξ), which quantifies the change in the number of moles of reactants and products.
- 😀 At equilibrium, the change in Gibbs free energy (ΔG) is zero, and the system reaches a state of minimal Gibbs free energy.
- 😀 The equilibrium constant (K) is derived from the Gibbs free energy change and is related to the fugacity (or partial pressures) of the reactants and products at equilibrium.
- 😀 The equilibrium constant depends on temperature and can change based on how the reaction is written (e.g., reversing the reaction or changing stoichiometric coefficients).
- 😀 The reaction advancement variable (ξ) is related to the change in moles of each component in a reaction, with the same value for both reactants and products.
- 😀 The system's energy and the Gibbs free energy for a reaction depend on factors like temperature, pressure, and the amounts of reactants and products involved.
Q & A
What defines a chemical equilibrium in a multi-component system?
-A chemical equilibrium in a multi-component system occurs when the mole fraction of each component remains constant over time. This means that the relative amount of each substance does not change, even though the reactions are still occurring in both directions.
What is the significance of Gibbs free energy in chemical equilibrium?
-Gibbs free energy (ΔG) is crucial for understanding chemical equilibrium because it indicates the spontaneity of a reaction. A negative ΔG means the reaction is spontaneous, while at equilibrium, ΔG equals zero, indicating no net change in the system.
How is the advance of a reaction (ξ) defined?
-The advance of a reaction (ξ) is a variable that quantifies the amount of reactants transformed into products during a chemical reaction. It is defined for all components involved in the reaction, with the same value for both reactants and products, and its dimensions are in moles.
What happens to Gibbs free energy as a reaction progresses?
-As a reaction progresses, the Gibbs free energy decreases until it reaches a minimum at equilibrium. The system tends to evolve towards the state where its Gibbs free energy is minimized.
What does it mean when ΔG equals zero?
-When ΔG equals zero, it indicates that the system is at equilibrium. No further net change occurs because the forward and reverse reactions happen at the same rate.
What is the role of temperature in determining the spontaneity of a process?
-Temperature plays a decisive role in determining whether a process is spontaneous. It affects both the enthalpy and entropy changes, influencing whether the Gibbs free energy decreases (spontaneous) or increases (non-spontaneous).
How does the equilibrium constant (K) relate to the Gibbs free energy?
-The equilibrium constant (K) is related to the Gibbs free energy by the equation ΔG° = -RT ln(K), where ΔG° is the standard Gibbs free energy change. At equilibrium, ΔG equals zero, leading to the relationship between K and the fugacities or partial pressures of the components.
What is the significance of the fugacity in the context of equilibrium?
-Fugacity is a measure of the effective pressure of a component in a mixture and is used to calculate the equilibrium constant. At equilibrium, the fugacity of each component in the system is related to the equilibrium constant, providing a way to determine the system's composition.
How does changing the stoichiometry of a reaction affect the equilibrium constant?
-Changing the stoichiometry of a reaction by multiplying the coefficients by a factor alters the equilibrium constant. The new equilibrium constant depends on the adjusted stoichiometry and temperature, but it remains related to the Gibbs free energy of the reaction.
Why does the equilibrium constant depend on temperature?
-The equilibrium constant depends on temperature because the standard Gibbs free energy of a reaction (ΔG°) is temperature-dependent. As temperature changes, the value of K changes, reflecting the shift in the equilibrium position.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)