SIFAT MATERI
Summary
TLDRThis educational video provides a detailed explanation of the physical and chemical properties of matter. It breaks down key concepts such as states of matter (solid, liquid, gas), melting and boiling points, solubility, density, hardness, elasticity, and electrical conductivity. The video also delves into chemical properties, focusing on reactions like flammability, reactivity, rusting, and decomposition. Through practical examples and a practice exercise, the viewer is encouraged to engage with the material to better understand the fundamental characteristics of substances, making chemistry more approachable and interesting.
Takeaways
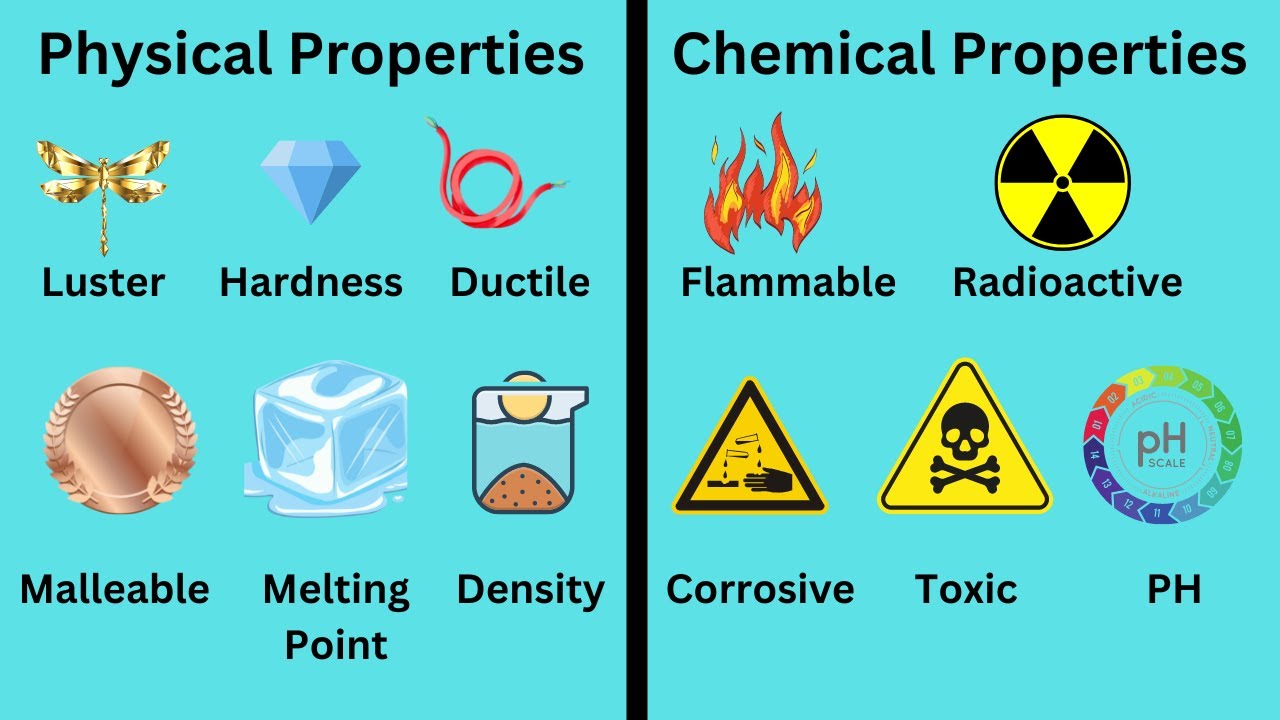
- 😀 Physical properties of matter can be measured by our senses and include characteristics like shape, size, and texture.
- 😀 Chemical properties of matter involve reactions that result in the formation of new substances, like rusting or burning.
- 😀 Matter exists in three main states: solid, liquid, and gas, each with distinct characteristics related to particle arrangement and movement.
- 😀 In solids, particles are tightly packed and cannot move, giving solids a fixed shape and volume.
- 😀 Liquids have particles that are close together but able to move, allowing them to take the shape of their container while maintaining a constant volume.
- 😀 In gases, particles are spread out and can move freely, so gases have neither a fixed shape nor a fixed volume.
- 😀 Melting point (or freezing point) refers to the temperature at which a solid turns into a liquid, while boiling point refers to when a liquid turns into a gas.
- 😀 Solubility refers to the maximum amount of a substance (like sugar) that can dissolve in a solvent (like water) at a given temperature.
- 😀 Density is a physical property that compares the mass of an object to its volume, and it varies across different substances.
- 😀 Hardness is a physical property that indicates how easily a material can be scratched or damaged by other objects.
- 😀 Chemical properties include flammability (how easily something burns), reactivity (how it reacts with other substances), and the potential for rusting or decomposition.
Q & A
What are the two main categories of properties of matter discussed in the script?
-The two main categories of properties of matter discussed are physical properties and chemical properties.
How are physical properties defined in the script?
-Physical properties are defined as characteristics that can be measured or observed using the senses. These include properties like shape, volume, and texture.
What are the three states of matter mentioned in the script, and how do their properties differ?
-The three states of matter mentioned are solid, liquid, and gas. Solids have tightly packed particles with a fixed shape and volume. Liquids have particles that are close together but can move, with a fixed volume but shape dependent on the container. Gases have particles that are far apart and move freely, with neither fixed shape nor volume.
What is the significance of melting and boiling points in understanding physical properties?
-Melting and boiling points are important physical properties because they indicate the temperatures at which a substance changes its state. The melting point is when a solid turns into a liquid, and the boiling point is when a liquid turns into a gas.
What does solubility refer to, and why is it considered a physical property?
-Solubility refers to the maximum amount of a substance that can dissolve in a solvent at a specific temperature. It is a physical property because it does not change the chemical composition of the substances involved.
How does the concept of density relate to physical properties?
-Density is a physical property that measures how much mass a substance has per unit of volume. It helps in understanding how tightly packed the particles of a substance are.
What is the role of hardness as a physical property?
-Hardness refers to a material's ability to resist being scratched or dented by other materials. It is an important physical property for identifying and comparing substances.
How is elasticity defined, and why is it important for understanding physical properties?
-Elasticity is the ability of a material to return to its original shape after being stretched or compressed. It is an important physical property because it determines how a material reacts to force.
What are conductors and insulators, and how do they differ?
-Conductors are materials that can easily conduct electricity and heat, such as metals. Insulators are materials that do not conduct electricity or heat well, such as wood and plastic.
What is the difference between physical and chemical properties based on the script?
-Physical properties describe characteristics that can be observed or measured without changing the chemical composition of a substance. Chemical properties, on the other hand, describe how a substance interacts with other substances, leading to a change in its chemical composition, such as combustion or rusting.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тариф5.0 / 5 (0 votes)