PROPIEDADES DE LA MATERIA. 26 ejercicios para practicar.
Summary
TLDRThis educational video script explores the properties of matter, essential for understanding the physical world. It distinguishes between general properties like mass, volume, and inertia, which all matter possesses, and specific properties that vary with the state of matter. Specific properties are further divided into physical, perceivable through senses like color and taste, and chemical, manifesting during composition changes. Examples include solubility, conductivity, and reactivity. The script aims to deepen the viewer's comprehension of these fundamental concepts.
Takeaways
- 🌐 Everything around us, from cells to computers, animals, water, and even air, is made up of matter.
- 🔍 Matter is anything that occupies space and has mass.
- 📏 Properties of matter are characteristics that help identify and describe it, divided into general and specific properties.
- 📚 General properties are common to all matter, including mass, weight, volume, inertia, porosity, elasticity, impenetrability, and divisibility.
- 📊 Mass is the amount of matter in an object, measurable in kilograms, and weight is the force of attraction by Earth's gravity, measured in newtons.
- 💧 Volume is the space an object occupies, commonly measured in liters for liquids and can be determined by water displacement for irregular solids.
- 🛴 Inertia is the resistance of an object to change its state of rest or motion, depending on its mass.
- 🕳️ Porosity refers to the empty spaces between particles within a material.
- 🔄 Elasticity is a material's ability to return to its original shape after a temporary deformation.
- 🚫 Impenetrability is the impossibility of two bodies occupying the same space at the same time.
- 🔁 Divisibility is the capacity of matter to be split into smaller portions.
- 🔬 Specific properties depend on the state of matter and are further divided into physical and chemical properties.
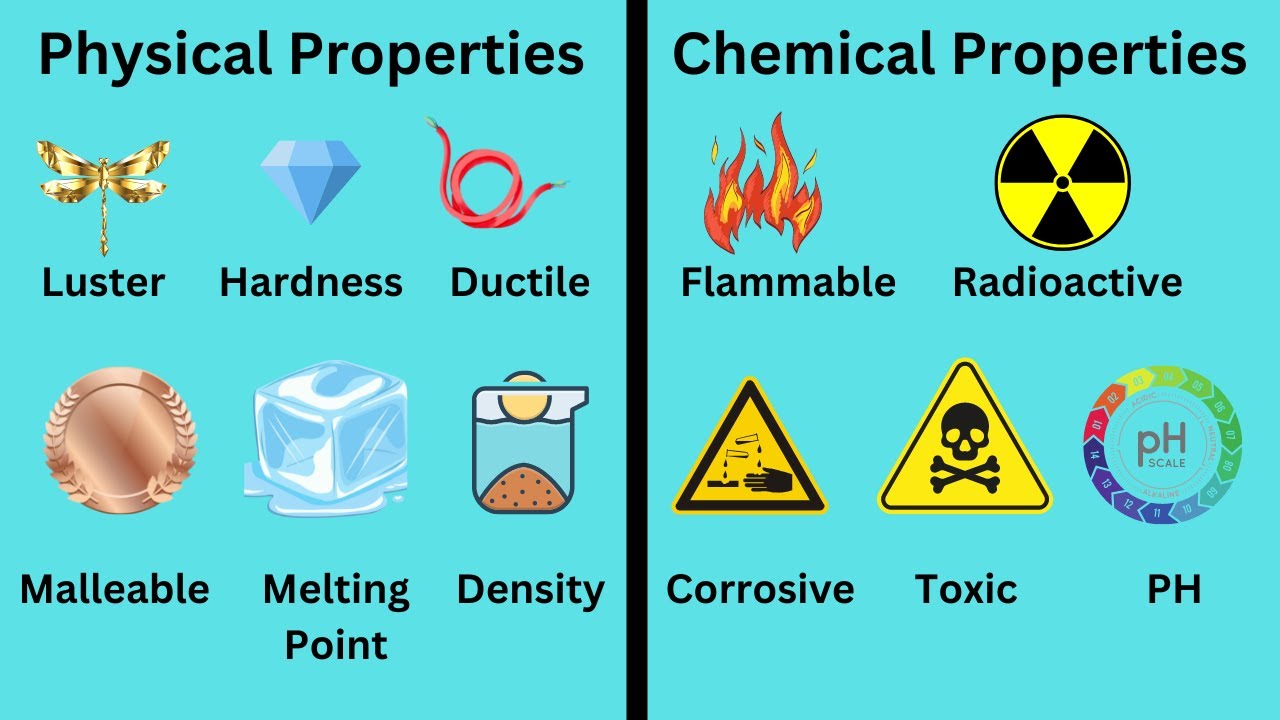
- 🌈 Physical properties can be observed without changing the substance's nature, such as color, taste, smell, sound, and texture.
- ⚖️ Hardness is a physical property measured on the Mohs scale of mineral hardness, ranging from talc (softest) to diamond (hardest).
- 🧵 Ductility is the ability of materials, like metals, to be drawn into thin wires.
- 📄 Malleability allows materials, such as aluminum foil, to be hammered into thin sheets.
- 💧 Density is the ratio of mass to volume of a substance, with water having a density of 1 g/mL and mercury much higher.
- 🌡️ Boiling point is the temperature at which a substance transitions from liquid to gas, while the melting point is the temperature for the transition from solid to liquid.
- 💧 Solubility is the property of certain substances to dissolve in a liquid at a specific temperature.
- 🔥 Conductivity refers to the ability of materials to transfer heat and electricity, with metals being good conductors.
- 🔥 Chemical properties manifest when matter changes its composition, such as reactivity, combustion, oxidation, and reduction.
Q & A
What is the definition of matter?
-Matter is anything that occupies space and has mass, including everything you see around you and even things like air that you cannot see.
What are the two main groups of properties of matter?
-The two main groups of properties of matter are general properties, which are common to all matter, and specific properties, which depend on the state of aggregation of the matter.
What is mass and how is it measured?
-Mass is the amount of matter contained in an object and is typically measured in kilograms using a scale or balance.
How is the force of gravity related to mass?
-The force of gravity is the force with which objects are attracted by the Earth's center, and it can be expressed in newtons when considering the value of gravity.
What is volume and how can it be measured?
-Volume is the space that a body occupies. It can be measured in liters for liquids, and for irregular bodies, the displacement method can be used by submerging the object in water and measuring the difference in volume.
What is inertia and how does it relate to mass?
-Inertia is the resistance of a body to change its state of rest or motion, and it depends on the mass of the object.
What is porosity and why is it significant?
-Porosity refers to the empty spaces that exist between the particles of a material, which can affect properties such as water absorption and density.
How is elasticity different from plasticity?
-Elasticity is the ability of a body to return to its original shape after a temporary deformation, whereas plasticity is the property of a material to undergo permanent deformation without breaking.
What does it mean for a material to be impenetrable?
-Impenetrability refers to the property of a material that prevents two bodies from occupying the same space at the same time.
What are the specific physical properties of matter?
-Specific physical properties of matter include organoleptic properties like color, taste, smell, and texture, as well as properties such as hardness, ductility, and malleability.
How is the hardness of minerals measured?
-The hardness of minerals is measured on the Mohs scale of mineral hardness, ranging from 1 (talc, which is easily scratched by a fingernail) to 10 (diamond, the hardest known natural material).
What is the difference between ductility and malleability?
-Ductility is the ability of certain materials to be drawn into a thin wire, while malleability is the ability to be hammered or rolled into thin sheets.
What is density and how does it relate to mass and volume?
-Density is the ratio of mass to volume for a substance. For example, the density of water is 1 g/cm³, meaning one milliliter of water weighs one gram.
What are the specific chemical properties of matter?
-Specific chemical properties of matter include reactivity, combustibility, oxidation, and reduction, which manifest when the matter changes its composition.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)