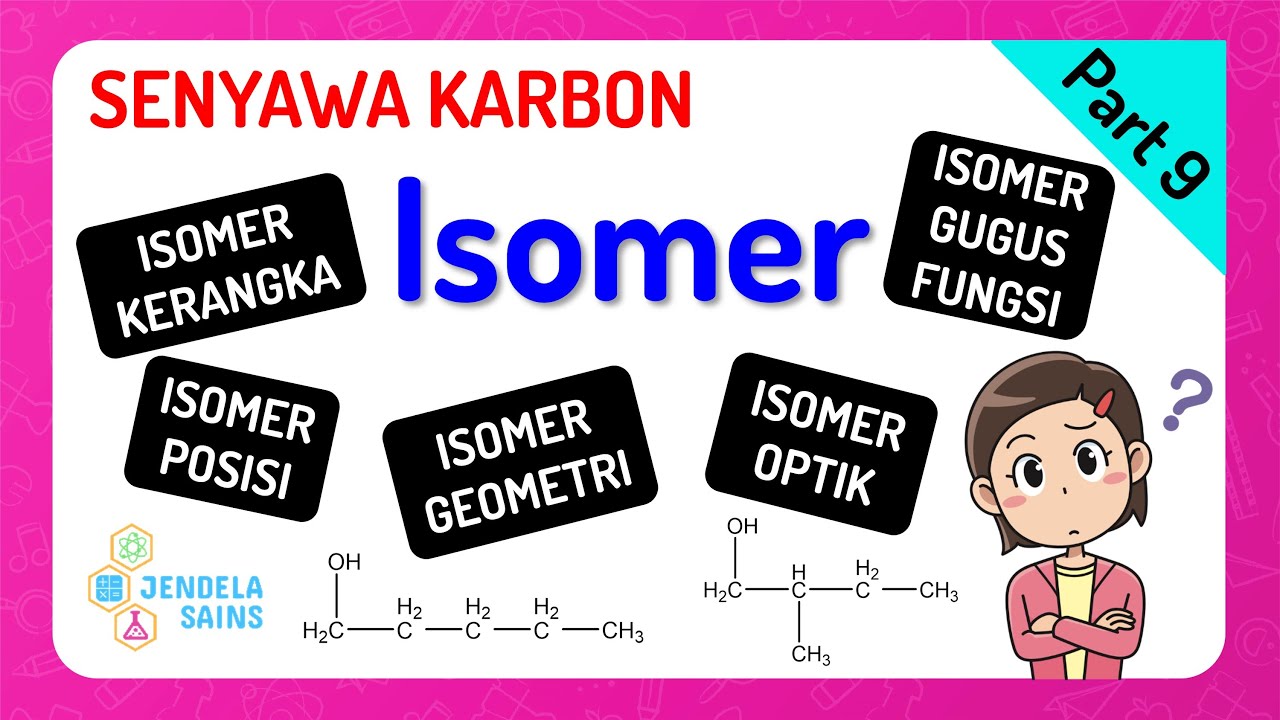
ISOMERIA PLANA
Summary
TLDRThis video explains the concept of isomerism in organic chemistry, focusing on the difference between compounds with the same molecular formula but different structures. The lesson covers two main types of isomerism: planar and spatial. It explores five types of planar isomerism—function, chain, position, and metamerism—as well as geometric and optical spatial isomerism. The video breaks down each concept with clear examples and offers practical tips for understanding isomers. Viewers are encouraged to stay engaged for a thorough understanding of these fundamental chemical concepts.
Takeaways
- 😀 Isomers are compounds that have the same molecular formula but different structural arrangements, resulting in distinct properties and names.
- 😀 Isomerism is divided into planar (constitutional) and spatial (stereo) types, each with specific subcategories.
- 😀 Planar isomerism includes types like functional group, chain, position, and metamerism, focusing on how the structure differs in terms of function, chain type, or position of substituents.
- 😀 Functional group isomerism occurs when compounds with the same molecular formula have different functional groups, like alcohols vs. aldehydes.
- 😀 Chain isomerism arises when the carbon chain is arranged differently, such as a straight chain vs. a branched chain or cyclic vs. open chain.
- 😀 Position isomerism occurs when the functional group or substituent is located at different positions on the carbon chain, as seen in alcohols with hydroxyl groups in different positions.
- 😀 Metamerism is a special case of position isomerism, where the atom or group moves within the same functional group but with a shift in position, like in amines or ketones.
- 😀 Tautomerism is a dynamic form of isomerism where two different forms (like keto-enol) exist in equilibrium, and both can interconvert.
- 😀 Understanding the structure and position of functional groups or atoms in organic compounds is key to identifying different types of isomerism.
- 😀 The video emphasizes the importance of knowing functional groups and carbon chain structures, as these form the basis for identifying isomerism in organic compounds.
Q & A
What are isomers?
-Isomers are compounds that have the same molecular formula but differ in their structural arrangement or spatial configuration. Despite having the same chemical composition, their structures and properties are different.
What is isomerism?
-Isomerism is the phenomenon where two or more compounds share the same molecular formula but differ in their structure or spatial arrangement. These compounds are called isomers.
What is the primary distinction between isomerism and different types of isomers?
-Isomerism is the general concept, while types of isomers refer to specific structural or spatial differences within compounds that share the same molecular formula. These include structural (planar) isomerism and spatial isomerism.
What is planar (structural) isomerism?
-Planar isomerism involves isomers that differ in their structural arrangements, which can be seen in the molecule's flat or planar structure. It includes subtypes like functional group isomerism, chain isomerism, positional isomerism, and metamerism.
What is functional group isomerism?
-Functional group isomerism occurs when two compounds have the same molecular formula but differ in the functional groups present in their structure. For example, alcohol and aldehyde can be functional isomers of the same molecular formula.
How does chain isomerism differ from positional isomerism?
-Chain isomerism involves isomers that differ in the type or structure of the carbon chain (e.g., straight chain vs. branched chain), while positional isomerism involves changes in the position of functional groups or double bonds along the carbon chain.
What is metamerism in organic chemistry?
-Metamerism is a special form of isomerism where the position of an atom like nitrogen or oxygen within the molecular chain changes, creating different structural isomers with the same molecular formula.
What is spatial isomerism?
-Spatial isomerism involves the three-dimensional arrangement of atoms or groups in space. This includes geometric isomerism (cis-trans) and optical isomerism (chirality), where isomers have different spatial orientations or can rotate plane-polarized light.
What is the difference between cis-trans isomerism and optical isomerism?
-Cis-trans isomerism, a form of geometric isomerism, occurs when the same bonds are arranged differently in space (e.g., cis vs. trans), while optical isomerism occurs when molecules are non-superimposable mirror images of each other, allowing them to rotate plane-polarized light.
What is tautomerism, and how does it differ from other types of isomerism?
-Tautomerism is a dynamic form of isomerism where two compounds exist in equilibrium, constantly converting from one form to another. This differs from other types of isomerism, where isomers are static and do not interconvert. A common example is keto-enol tautomerism.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео
5.0 / 5 (0 votes)