A Level Chemistry Revision "Structural Isomers"
Summary
TLDRThis video introduces the concept of structural isomers, molecules with the same molecular formula but different structural arrangements. It explains various examples, including alkanes like butane and pentane, as well as alkenes such as pent-1-ene. The video also covers chlorinated compounds, highlighting their structural isomers. Key categories of structural isomers are discussed: functional group isomers, chain isomers, and positional isomers, each with distinct characteristics. The lesson aims to provide a solid understanding of structural isomerism, a vital concept in organic chemistry.
Takeaways
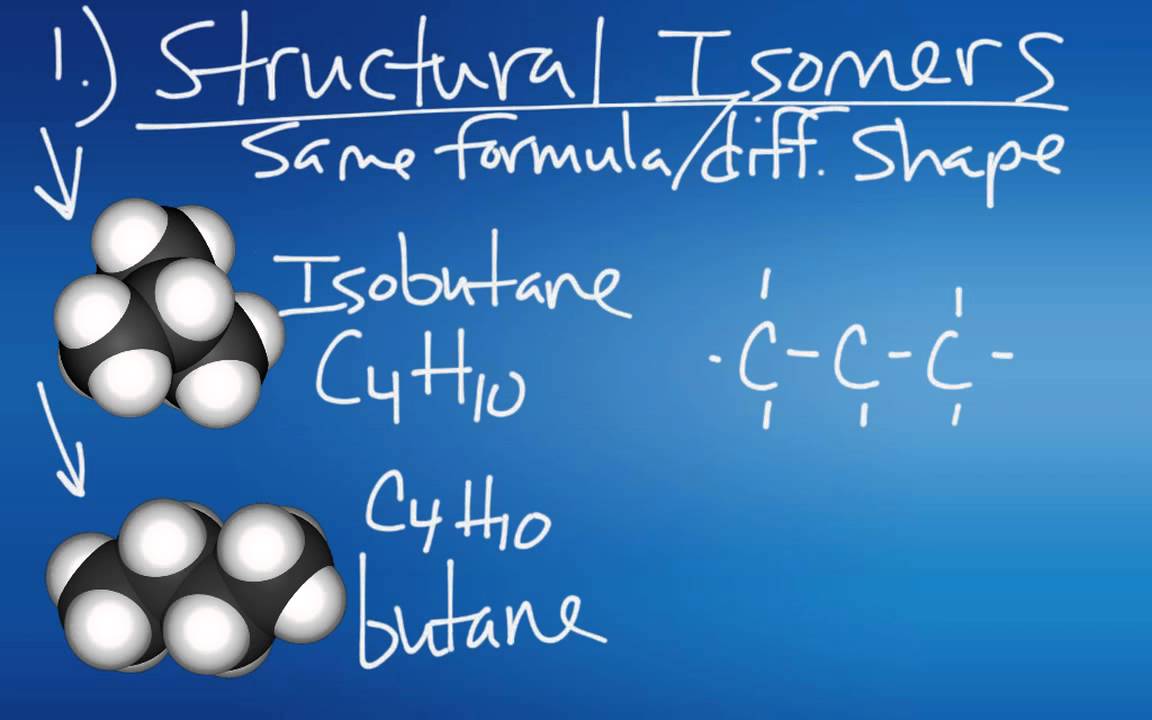
- 😀 Structural isomers are molecules with the same molecular formula but different structural formulae.
- 😀 Butane and methylpropane are examples of structural isomers, both having the molecular formula C4H10 but different structures.
- 😀 Pentane (C5H12) has three structural isomers: pentane itself, methylbutane, and dimethylpropane.
- 😀 The name of methylbutane does not include a number because the methyl group is always attached to carbon 2.
- 😀 'Ethylpropane' is an incorrect name for a structural isomer of pentane; the correct name is methylbutane.
- 😀 Alkenes like pent-1-ene (C5H10) also have structural isomers, such as pent-1-ene and pent-2-ene, by shifting the position of the double bond.
- 😀 Cycloalkanes can be structural isomers of alkenes, although the video focused on alkenes only to simplify the examples.
- 😀 One chlorobutane has four structural isomers, including one chlorobutane and two chlorobutane, plus one chloromethylpropane.
- 😀 Structural isomers can sometimes differ in their functional groups, as seen in the example of ethanoic acid and methyl methanoate.
- 😀 Structural isomers can be categorized into functional group isomers, chain isomers, and positional isomers, based on differences in functional groups or the arrangement/position of atoms.
Q & A
What are structural isomers?
-Structural isomers are molecules that have the same molecular formula but different structural formulae, meaning their atoms are arranged differently.
Give an example of two structural isomers.
-Butane (C4H10) and methylpropane (C4H10) are structural isomers. They have the same molecular formula but different structures.
What is the molecular formula for pentane and how many structural isomers can it form?
-Pentane has the molecular formula C5H12, and it can form three structural isomers: pentane, methylbutane, and dimethylpropane.
Why is 'ethylpropane' an incorrect name for a structural isomer of pentane?
-'Ethylpropane' is incorrect because the longest carbon chain in the molecule is four carbons long, which should be named 'methylbutane'. The name 'ethylpropane' would imply a chain of three carbons.
What are the two structural isomers of pent-1-ene?
-The two structural isomers of pent-1-ene are pent-1-ene and pent-2-ene. These differ by the position of the double bond.
How does moving the methyl group in methylbutane affect the isomer?
-Moving the methyl group in methylbutane to different carbon atoms can create different structural isomers, such as 2-methylbut-1-ene or 3-methylbut-1-ene.
What are the four structural isomers of one-chlorobutane?
-The four structural isomers of one-chlorobutane are one-chlorobutane, two-chlorobutane, one-chloromethylpropane, and two-chloromethylpropane.
What is the misconception about a fifth structural isomer for chloromethylpropane?
-The misconception is that placing a chlorine atom on the methyl group of methylpropane creates a fifth isomer, but this is actually just a different drawing of one-chloromethylpropane.
What are functional group isomers, and can you give an example?
-Functional group isomers are molecules with the same molecular formula but different functional groups. An example is ethanoic acid and methyl methanoate.
What are the three categories of structural isomers according to the AQA specification?
-The three categories of structural isomers are functional group isomers (different functional groups), chain isomers (different hydrocarbon chain arrangements), and positional isomers (different positions of the functional group on the chain).
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)