What is an Atom -Basics for Kids
Summary
TLDRIn this video, an animated atom explains the basic concepts of atoms and molecules. The atom describes how atoms are the smallest indivisible units of matter, comparing them to letters in words. It explains the structure of an atom, including the nucleus, protons, neutrons, and orbiting electrons, and how atoms form molecules by sharing electrons. Using water as an example, the atom shows how hydrogen and oxygen atoms bond to create water molecules. The video emphasizes how atoms are fundamental to everything around us, encouraging viewers to learn more about this essential concept.
Takeaways
- 🔬 Atoms are so small that they can only be seen under a special microscope.
- ⚛️ The word 'atom' means 'uncuttable' or 'indivisible' in Greek.
- 🍏 Just like cutting an apple into smaller pieces, you reach a point where matter can't be divided further, which is called an atom.
- 🔗 Everything around us is made up of atoms, including molecules, which are composed of atoms.
- 🔠 Just as words are made up of letters, molecules are made up of atoms, which are the smallest unit of matter.
- 💧 Water is made up of molecules consisting of two hydrogen atoms and one oxygen atom (H2O).
- 💡 The nucleus of an atom is positively charged, containing protons (positive charge) and neutrons (no charge), while electrons (negative charge) orbit around it.
- 🌀 The total positive charge of the nucleus is balanced by the total negative charge of electrons, making the atom neutral overall.
- ⚙️ Electrons orbit in shells around the nucleus, and each shell can hold a fixed number of electrons (2 in the first, 8 in the second, etc.).
- 🤝 Atoms bond with other atoms by sharing electrons in their outermost shell, called the valence shell, to form molecules like water.
Q & A
What is the meaning of the word 'atom'?
-The word 'atom' comes from a Greek word meaning 'that which can't be cut' or 'indivisible,' referring to something that cannot be divided further.
Why can't atoms be seen with the naked eye?
-Atoms are extremely small and can only be seen using a special type of microscope, called a machine in the script, because they are too tiny for the naked eye to detect.
How does the script explain the relationship between atoms and molecules?
-The script explains that everything is made up of atoms. Molecules are formed when atoms join together, similar to how letters form words.
What is the smallest part of a sentence according to the analogy in the script?
-The smallest part of a sentence is a letter, just as the smallest part of matter is an atom.
What are the components of an atom's nucleus?
-The nucleus of an atom is made up of protons and neutrons. Protons carry a positive charge, while neutrons have no charge.
Why is an atom neutral in charge?
-An atom is neutral because the positive charge of the protons in the nucleus is balanced by the negative charge of the electrons that orbit around the nucleus.
What keeps electrons from running away from the nucleus?
-Electrons are attracted to the positively charged protons in the nucleus, which prevents them from escaping due to the balance of these opposite charges.
How many electrons can each shell of an atom hold?
-The first shell can hold up to 2 electrons, the second up to 8, the third up to 18, and the fourth up to 32 electrons.
What are 'valence electrons'?
-Valence electrons are the electrons in the outermost shell of an atom, called the valence shell. These electrons are involved in forming bonds with other atoms.
How does the script explain the formation of a water molecule?
-A water molecule is formed when an oxygen atom, which needs two more electrons to fill its valence shell, bonds with two hydrogen atoms, each sharing one electron. This bond allows both the oxygen and hydrogen atoms to fill their outer shells.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Perbedaan Atom, Unsur, Senyawa, Molekul dan Ion | KIMIA KELAS 10

Ikatan Kimia (8) | Cara Menentukan Molekul Polar dan Non Polar | Kimia Kelas 10

Conceitos básicos [Módulo 01_Aula 04]
ALEKS: Writing Lewis structures for a molecule with one central atom and no octet rule exceptions

Prótons, elétrons, nêutrons e massa Fácil- como calcular e exemplos
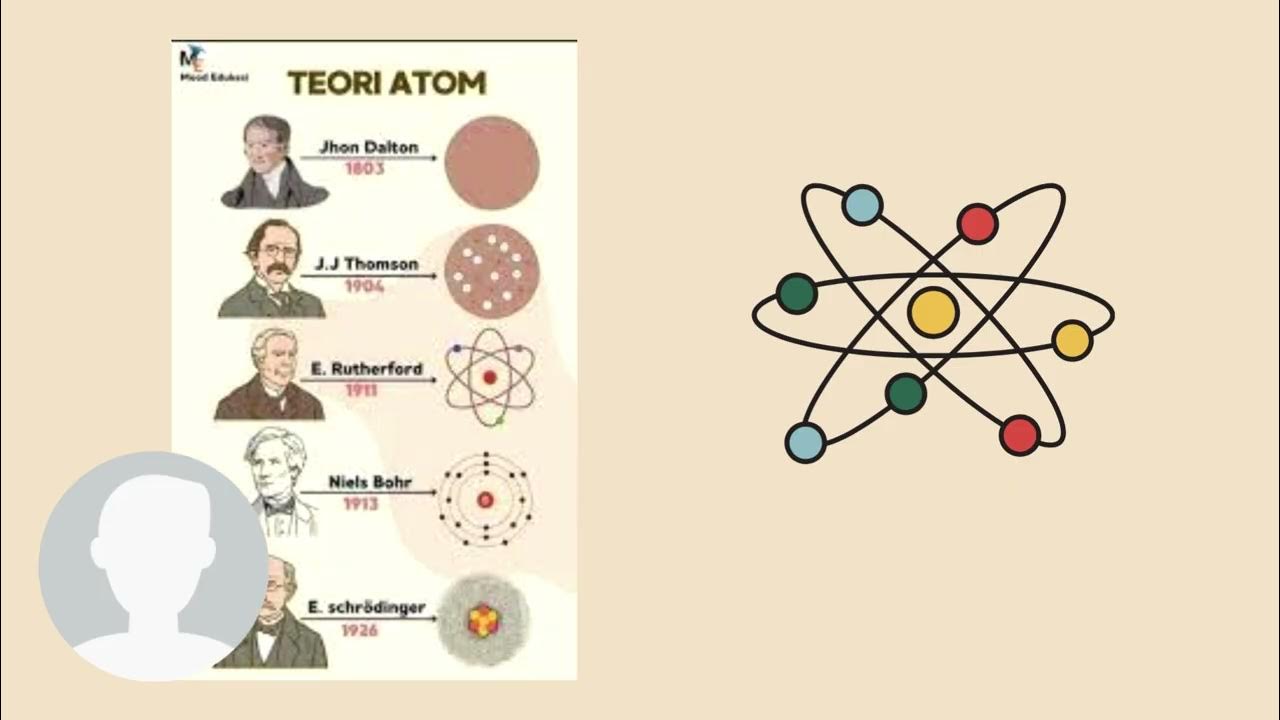
stuktur atom
5.0 / 5 (0 votes)
