How to Balance Chemical Equations in 5 Easy Steps: Balancing Equations Tutorial
Summary
TLDRThis video provides a step-by-step guide to balancing chemical equations in an easy-to-follow manner. It emphasizes counting atoms on both sides of the equation, adjusting coefficients (without changing subscripts), and tackling more complex equations with polyatomic ions. Key examples include balancing sulfur and oxygen atoms, working through hydrogen and oxygen molecules, and simplifying tricky equations by treating polyatomic ions as single units. The importance of practice to master speed and accuracy in balancing equations is highlighted. A clear, engaging tutorial designed to build understanding and confidence.
Takeaways
- 🔢 **Count Atoms**: Begin by counting the atoms of each element on both sides of the equation.
- 📝 **Assume '1'**: If no coefficient is written, assume it to be '1'.
- 🚫 **Don't Change Subscripts**: Only change coefficients; never alter the subscripts of elements.
- ⚖️ **Balance Each Element**: Ensure the number of atoms for each element is equal on both sides.
- 🔄 **Adjust Coefficients**: Correctly adjust the coefficients to balance the atoms of each element.
- 🤔 **Check Oxygen First**: Often, balancing oxygen can be straightforward and a good starting point.
- 🔄 **Recheck After Changes**: After adjusting coefficients, recheck to ensure all elements are balanced.
- 🌐 **Treat Polyatomic Ions as Units**: Consider polyatomic ions as single units to simplify the balancing process.
- 📚 **Practice for Expertise**: Regular practice is key to improving speed and accuracy in balancing chemical equations.
- 👨🏫 **Guidance from Dr. B**: The script is a teaching tool provided by Dr. B to help viewers understand the balancing process.
Q & A
What is the first step in balancing a chemical equation?
-The first step is to count the atoms on each side of the equation and ensure that the numbers of each type of atom are equal on both sides.
What is the rule for balancing chemical equations?
-The rule for balancing chemical equations is that you can only change the coefficients (numbers in front of the atoms), not the subscripts (numbers after the atoms).
How do you treat elements without a coefficient written in front of them?
-If there is no coefficient written in front of an element, it is assumed to be '1'.
What did the example demonstrate about balancing sulfur and oxygen atoms?
-The example showed that by changing the coefficient of sulfur on the product side to eight, and then adjusting the coefficient of oxygen to eight, both sulfur and oxygen atoms were balanced on both sides of the equation.
Why is it important to only change coefficients when balancing equations?
-Changing only the coefficients ensures that the chemical equation remains chemically accurate, as altering subscripts would imply changing the structure of the molecules involved.
What is a polyatomic ion and how does it simplify balancing equations?
-A polyatomic ion is a group of atoms that act as a single ion with a specific charge. Treating polyatomic ions as one entity simplifies balancing equations because you can balance the entire ion instead of individual atoms.
How did the script demonstrate balancing an equation with polyatomic ions?
-The script showed that by treating SO4 and NO3 as single units, the equation could be balanced by adjusting the coefficients to ensure that the number of each polyatomic ion was equal on both sides.
What is a common mistake to avoid when counting atoms in a chemical equation?
-A common mistake is not counting all atoms, especially those that are part of polyatomic ions or groups. Each atom must be counted accurately to ensure the equation is balanced.
How does practice help in balancing chemical equations?
-Practice helps in gaining speed and expertise in balancing chemical equations by becoming familiar with the process and recognizing patterns in different types of equations.
What is the final advice given by Dr. B in the script?
-Dr. B advises that the more you practice balancing chemical equations, the easier it becomes, emphasizing the importance of practice for mastering the skill.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Penyetaraan Persamaan Reaksi Kimia dengan Cepat- Kimia Kelas 10

Balancing Chemical Equations Practice Problems
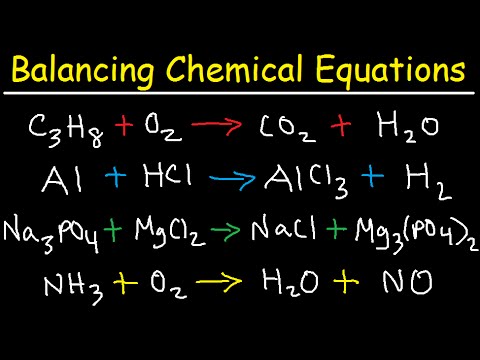
Introduction to Balancing Chemical Equations

Penyetaraan Reaksi Metode Aljabar

Stoikiometri (5) | Perhitungan Dalam Persamaan Reaksi | Kimia Kelas 10

Stoikiometri Larutan • Part 1: Persamaan Ion dan Reaksi Penggaraman
5.0 / 5 (0 votes)
