Common polyatomic ions | Atoms, compounds, and ions | Chemistry | Khan Academy
Summary
TLDRThis educational video script introduces viewers to common polyatomic ions, essential for general chemistry classes. It explains the naming conventions and structures of cations like the Ammonium ion and anions such as Acetate, Cyanide, Hydroxide, and Permanganate. The script emphasizes the significance of suffixes like 'ate' and 'ite' to indicate the number of oxygen atoms. Examples include the differentiation between Nitrate and Nitrite, as well as the addition of prefixes like 'per-' and 'hypo-' to denote variations in oxygen count. The video also covers ions like Sulfate, Carbonate, Phosphate, and Chromate, highlighting how adding H+ ions affects their charges and names, such as Hydrogen Sulfate and Bicarbonate. The script serves as a valuable study aid for memorizing these complex ions.
Takeaways
- 🔬 Memorizing polyatomic ions is crucial for general chemistry classes.
- 🌐 Cation NH4+ is known as the Ammonium ion, carrying a positive charge.
- ⚛️ Anions include Acetate (CH3COO-), Cyanide (CN-), Hydroxide (OH-), and Permanganate (MnO4-).
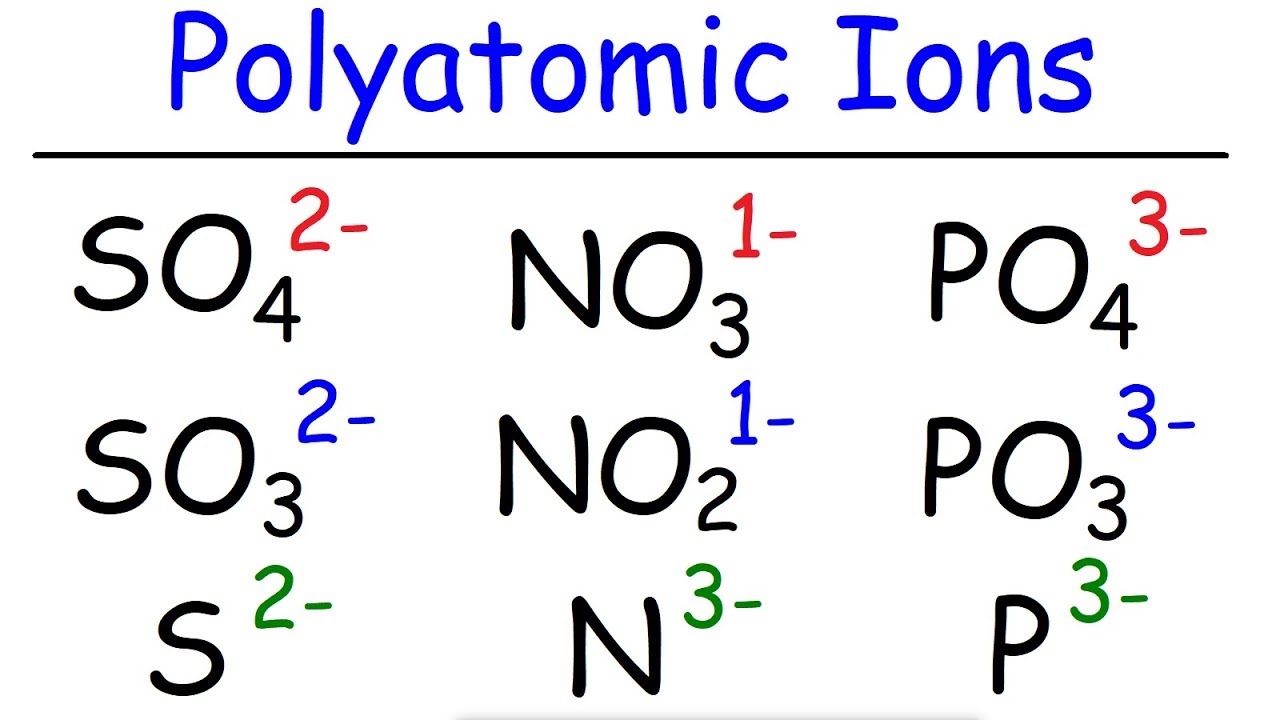
- 🔍 The suffix 'ate' in polyatomic ions like Nitrate (NO3-) indicates more oxygens compared to 'ite' in Nitrite (NO2-).
- 🌿 The prefix 'per' in Perchlorate (ClO4-) adds one more oxygen compared to Chlorate (ClO3-).
- 🧪 Hypochlorous acid (HClO) is formed by removing one oxygen from Chlorite (ClO2-), reflecting the 'hypo' prefix.
- 📚 Sulfate (SO42-) has four oxygens, while Sulfite (SO32-) has three, illustrating the 'ite' ending.
- 🌐 Hydrogen Sulfate (HSO4-) is formed by adding an H+ to Sulfate, changing the charge from -2 to -1.
- 🔋 Adding H+ to Carbonate (CO32-) forms Hydrogen Carbonate (HCO3-), also known as Bicarbonate.
- 🧬 Phosphate (PO43-) becomes Hydrogen Phosphate (HPO42-) upon adding an H+, and further addition forms Dihydrogen Phosphate (H2PO4-).
Q & A
What is the name of the positively charged ion with the formula NH4+?
-The positively charged ion with the formula NH4+ is called the Ammonium ion.
What does the prefix 'per-' signify in the context of polyatomic ions?
-The prefix 'per-' signifies one more oxygen atom than the base polyatomic ion.
How do you differentiate between 'Nitrate' and 'Nitrite' based on their chemical formulas?
-Nitrate is NO3- and has three oxygen atoms, while Nitrite is NO2- and has two oxygen atoms.
What is the difference between 'ate' and 'ite' suffixes in polyatomic ions?
-The 'ate' suffix indicates more oxygen atoms, while the 'ite' suffix indicates fewer oxygen atoms in the ion.
What is the chemical formula for the Hypochlorite ion?
-The chemical formula for the Hypochlorite ion is ClO-.
How does the addition of an H+ ion to Sulfate change its formula and name?
-When an H+ ion is added to SO4^2- (Sulfate), it becomes HSO4- and is called the Hydrogen Sulfate ion, also known as Bisulfate.
What is the name of the polyatomic ion with the formula CO3^2-?
-The polyatomic ion with the formula CO3^2- is called Carbonate.
What is the relationship between Phosphate and Hydrogen Phosphate in terms of their chemical formulas?
-Phosphate has the formula PO4^3-, and when an H+ ion is added, it becomes HPO4^2-, which is called Hydrogen Phosphate.
What is the name of the polyatomic ion with the formula CrO4^2-?
-The polyatomic ion with the formula CrO4^2- is called Chromate.
How do you determine the number of oxygen atoms in a polyatomic ion when given its name with the 'ite' or 'ate' suffix?
-The 'ite' suffix indicates a number of oxygen atoms that is one less than the 'ate' suffix for the corresponding ion.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Naming Polyatomic Ion Compounds With Transition Metals (31)

How to Name Acids: Examples and Practice

Writing Formulas with Polyatomic Ions

How to Write the Formula for Ionic Compounds with Polyatomic Ions
How to Memorize The Polyatomic Ions - Formulas, Charges, Naming - Chemistry
From Novice to Expert: Naming Chemicals Made Easy
5.0 / 5 (0 votes)
