Naming Polyatomic Ion Compounds With Transition Metals (31)
Summary
TLDRThis educational video script delves into the intricacies of naming compounds with polyatomic ions, a crucial concept in chemistry. It guides viewers through the process of identifying and naming these compounds, whether starting from a name or a formula. The script emphasizes the importance of memorizing common polyatomic ions and provides a systematic approach to handle complex scenarios, including those involving transition metals. With a touch of humor, it encourages viewers to practice and apply these naming rules to solidify their understanding of ionic compounds.
Takeaways
- 🧪 The video discusses the importance of understanding polyatomic ions in chemistry, emphasizing their prevalence in ionic compounds.
- 📚 It outlines the steps for naming compounds containing polyatomic ions, either from the name to the formula or vice versa.
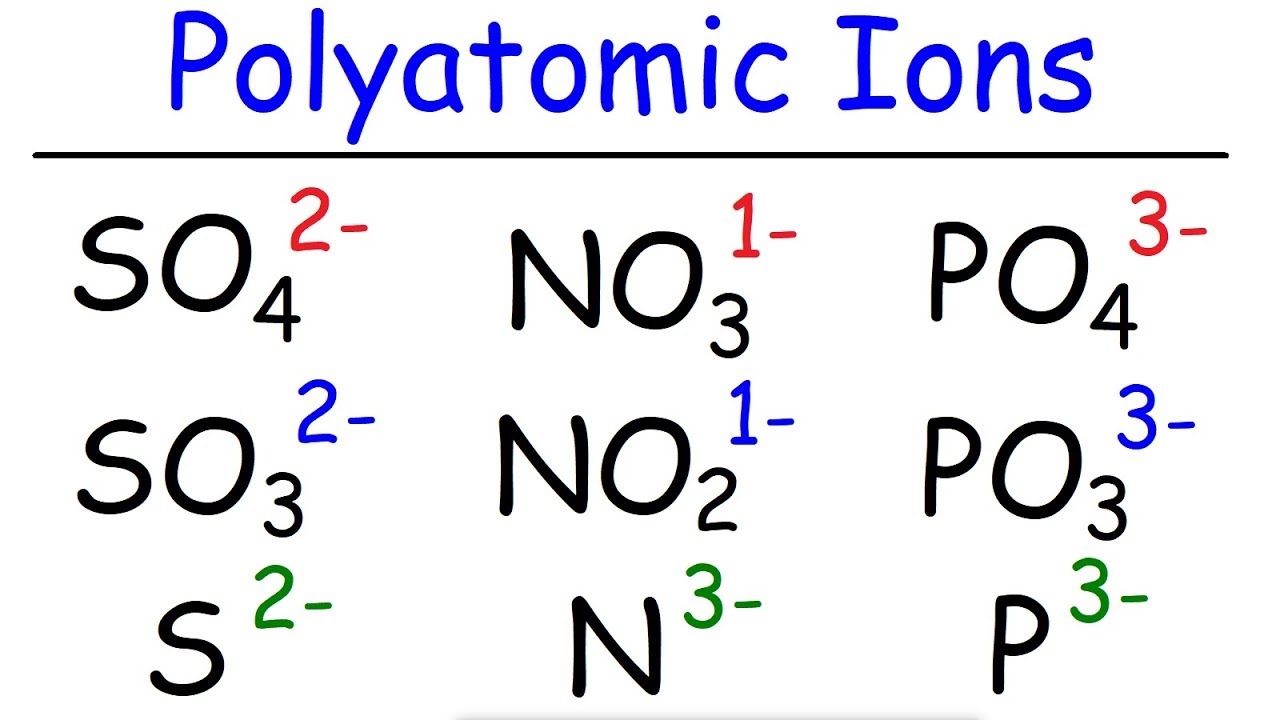
- 📝 The script advises viewers to have a list of common polyatomic ions handy for easy reference when naming compounds.
- 🔍 It explains the process of identifying polyatomic ions in a compound by looking for multiple different kinds of atoms.
- 📐 The video provides a method for naming ionic compounds with polyatomic ions, including the use of parentheses to denote the start and end of a polyatomic ion.
- 🔄 It highlights the exception of ammonium, which is a polyatomic cation and is named first, followed by the anion ending in 'ide'.
- 📖 The script covers the advanced topic of ionic compounds containing both polyatomic ions and transition metals, detailing the steps for their naming.
- 📝 It stresses the importance of correctly using parentheses to group polyatomic ions together to avoid confusion about the number of ions present.
- 🧩 The video includes practice examples to help viewers apply the naming rules for ionic compounds with polyatomic ions.
- 🎓 Lastly, it summarizes the general rules for naming ions, categorizing them into three cases: normal metal with non-metal, transition metal with non-metal, and metal with polyatomic ion.
Q & A
What is the main focus of the video script?
-The main focus of the video script is to teach viewers how to name compounds containing polyatomic ions, either from the name to the formula or the formula to the name, and to summarize the steps for naming ionic compounds in general.
Why are polyatomic ions important in chemistry?
-Polyatomic ions are important in chemistry because they are groups of two or more non-metal atoms that act as a single ion with a specific charge, and they are commonly found in many chemical compounds.
What is the first step in naming compounds with polyatomic ions?
-The first step in naming compounds with polyatomic ions is to name the compound with the same rules as before, which involves identifying the metal and non-metal elements, with the non-metal often being the polyatomic ion.
Why is it necessary to write down the names of common polyatomic ions?
-It is necessary to write down the names of common polyatomic ions because they are frequently used in naming compounds and having them readily available helps in quickly and accurately identifying and naming these ions.
What is the 'cookie' analogy used in the script for?
-The 'cookie' analogy is used to explain the importance of using parentheses around polyatomic ions to indicate the start and end of the ion, which helps in correctly interpreting the formula and charge balance of the compound.
How does the script differentiate between naming ionic compounds with transition metals and those without?
-The script differentiates by stating that if there is a transition metal, it should be named first with a Roman numeral in parentheses to indicate its charge, followed by the anion, which ends in 'ide'. For non-transition metals, the cation is named first followed by the polyatomic ion.
What is the significance of the 'drop and swap' method mentioned in the script?
-The 'drop and swap' method is significant as it helps in balancing the charges in the compound by placing the appropriate numerical charges on the cation and anion to ensure the overall charge of the compound is neutral.
What is the role of parentheses when naming polyatomic ions in compounds?
-Parentheses are used to group the entire polyatomic ion together, which is crucial for indicating the correct number of atoms within the ion and for distinguishing between multiple ions in the compound.
Why is it important to recognize when a compound contains both a transition metal and a polyatomic ion?
-Recognizing when a compound contains both a transition metal and a polyatomic ion is important because it requires combining the naming conventions for both, ensuring the correct representation of the compound's structure and charge.
How does the script handle the exception of ammonium in polyatomic ion naming?
-The script handles the exception of ammonium by stating that it is the only polyatomic cation and should always be named first, followed by the anion. If paired with another polyatomic ion, the anion's name is used without converting it to an 'ide' ending.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)