Ligação Covalente - Brasil Escola
Summary
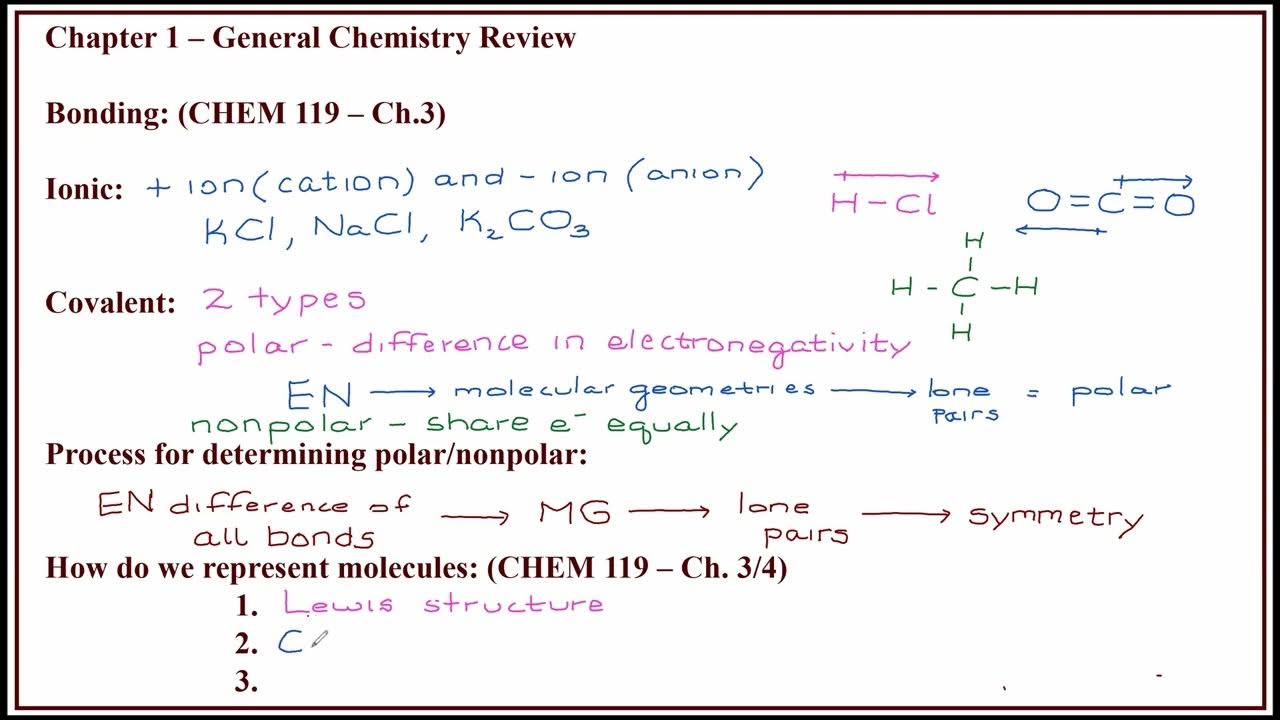
TLDRIn today's chemistry lesson, the focus is on covalent bonding, contrasting with ionic bonds. Covalent bonds, as explained, involve electron sharing between atoms, unlike ionic bonds which are characterized by electron transfer. The video uses the example of Cl2 to illustrate how atoms share electrons to achieve stability, following the octet rule. It simplifies the concept with a game lending analogy and discusses different types of covalent bonds, such as single, double, and triple bonds. The lesson also covers molecular formulas, electronic formulas, and structural formulas. An exercise from the ENEM (Brazilian college entrance exam) is used to apply these concepts, focusing on molecules with expanded octets. The video concludes with an invitation to engage with the content and explore further topics.
Takeaways
- 🔬 Covalent bonds are based on the sharing of electron pairs between atoms, differing from ionic bonds which involve the transfer of electrons.
- 📚 The term 'covalence' refers to the sharing of electrons, which is fundamental to covalent bonding.
- 🧩 In a covalent bond, atoms with a tendency to gain electrons share electron pairs to achieve stability, following the octet rule.
- 🌐 An example given is Cl2, where each chlorine atom shares one electron to complete its valence shell, resulting in a double bond between the two chlorine atoms.
- 🎮 A relatable analogy is borrowing a video game from a friend while lending one of yours, symbolizing the mutual sharing in covalent bonds.
- 🔗 Covalent bonds can be single, double, or triple, depending on the number of electron pairs shared between atoms.
- 📐 The script explains that covalent bonds help atoms achieve a noble gas electron configuration, typically by sharing electrons to complete their octets.
- 🚫 The concept of 'expanded octet' is introduced, where atoms with empty d orbitals can accommodate more than eight electrons, thus deviating from the traditional octet rule.
- 📚 The script clarifies that while BF3 and NH3 adhere to the octet rule, PCl5 is an example of an expanded octet, with the phosphorus atom stabilizing with more than eight electrons.
- 📝 The video script is educational, aiming to help students understand the basics of covalent bonding to solve chemistry problems, such as identifying expanded octets in molecules.
- 👨🏫 The instructor encourages engagement, suggesting that viewers like the video, comment with questions or suggestions, and subscribe for more educational content.
Q & A
What is the main topic discussed in the script?
-The main topic discussed in the script is covalent bonding in chemistry, focusing on the concept of electron sharing between atoms to form chemical bonds.
How does covalent bonding differ from ionic bonding?
-Covalent bonding involves the sharing of electron pairs between atoms, unlike ionic bonding which involves the transfer of electrons from one atom to another, typically between a metal and a non-metal.
What is the basis of covalent bonding?
-The basis of covalent bonding is the sharing of electron pairs to achieve stability, often by fulfilling the octet rule where atoms share electrons to complete their outer electron shell.
What is the role of the octet rule in covalent bonding?
-The octet rule plays a significant role in covalent bonding as it states that atoms tend to share, gain, or lose electrons to achieve a stable electron configuration, typically having eight electrons in their valence shell, similar to noble gases.
Can you explain the example of Cl2 given in the script?
-The script uses Cl2 as an example to illustrate covalent bonding. Each chlorine atom has seven valence electrons and shares one with the other to complete its octet, resulting in a mutual sharing of one electron pair between the two chlorine atoms.
What are the different types of covalent bonds mentioned in the script?
-The script mentions three types of covalent bonds: single bond (one shared electron pair), double bond (two shared electron pairs), and triple bond (three shared electron pairs).
How are covalent bonds represented in chemical structures?
-Covalent bonds are represented in chemical structures by lines connecting the bonded atoms. A single line represents a single bond, a double line represents a double bond, and a triple line represents a triple bond.
What are the three main ways to represent a molecule mentioned in the script?
-The script mentions three main ways to represent a molecule: molecular formula, electronic formula (also known as Lewis structure), and structural formula.
What is the significance of the term 'expanded octet' in the context of the script?
-The term 'expanded octet' refers to a situation where an atom in a molecule can accommodate more than eight electrons in its valence shell, typically by using empty d orbitals, which is an exception to the standard octet rule.
Which of the given molecules in the exercise (BF3, NH3, PCl5, BeH2, and Al3) exhibits an expanded octet?
-Among the given molecules, PCl5 exhibits an expanded octet because the phosphorus atom in PCl5 can accommodate more than eight electrons in its valence shell.
What is the difference between covalent and coordinate covalent bonding as briefly mentioned in the script?
-While the script does not deeply explore the difference, covalent bonding typically involves equal sharing of electrons, whereas coordinate covalent bonding involves the donation of a lone pair of electrons from one atom to another, which contributes to the formation of a covalent bond without providing an electron itself.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тариф5.0 / 5 (0 votes)