Semaglutide, Chronic Kidney Disease, and Diabetes | NEJM
Summary
TLDRA recent trial has demonstrated that semaglutide, a glucagon-like peptide 1 receptor agonist, significantly reduces the risk of major kidney disease events in patients with type 2 diabetes and chronic kidney disease (CKD). Conducted as a randomized, controlled, double-blind study involving 3533 adults, the participants received weekly subcutaneous semaglutide or placebo. Over a median follow-up of 3.4 years, the semaglutide group showed a 24% risk reduction in major kidney events compared to the placebo group. Additionally, the decline in kidney function was slower in the treatment group, and serious adverse events were less frequent, highlighting the potential of semaglutide in managing kidney health in this high-risk population.
Takeaways
- 💉 Semaglutide is a glucagon-like peptide 1 receptor agonist used for treating type 2 diabetes.
- 🔍 A new trial has been conducted to assess the effects of semaglutide on kidney outcomes in patients with chronic kidney disease (CKD).
- 📊 The trial was a randomized, controlled, double-blind study involving 3533 adults with type 2 diabetes and CKD.
- 💡 Participants were assigned to receive weekly subcutaneous semaglutide or placebo injections.
- 🏥 The primary outcome measured was major kidney disease events, which included kidney failure onset, a significant eGFR reduction, or kidney/cardiovascular-related death.
- ⏱ The median follow-up duration for the trial was 3.4 years.
- 📉 Semaglutide reduced the risk of major kidney disease events by 24% compared to the placebo group.
- 📊 The semaglutide group experienced 5.8 major kidney disease events per 100 patient-years, versus 7.5 in the placebo group.
- 📉 The decline in kidney function was slower in the semaglutide group compared to the placebo group.
- 🚑 Serious adverse events were less common among those who received semaglutide.
- 📝 The study concludes that semaglutide is beneficial for patients with type 2 diabetes and high-risk CKD, reducing the risk of major kidney disease events.
- 🔗 Full trial results can be accessed on NEJM.org for further details.
Q & A
What is the main focus of the trial mentioned in the transcript?
-The main focus of the trial is to assess the effects of semaglutide on major kidney outcomes in patients with type 2 diabetes who also have chronic kidney disease (CKD).
What was the nature of the trial involving semaglutide and CKD patients?
-The trial was a randomized, controlled, double-blind study involving 3533 adults with type 2 diabetes and CKD.
What were the two treatment groups in the trial?
-The two treatment groups were one receiving weekly subcutaneous semaglutide and the other receiving a placebo.
What was the primary outcome measured in the trial?
-The primary outcome was major kidney disease events, which is a composite of kidney failure onset, a 50% or greater reduction in eGFR from baseline, or death from kidney-related or cardiovascular causes.
What was the median follow-up period for the trial?
-The median follow-up period for the trial was 3.4 years.
How did the semaglutide group compare to the placebo group in terms of major kidney disease events?
-The semaglutide group experienced 5.8 major kidney disease events per 100 patient-years of follow-up, compared to 7.5 events per 100 patient-years in the placebo group, indicating a 24% risk reduction.
What was the secondary outcome measured in the trial?
-The secondary outcome was the decline in kidney function, which was slower in the semaglutide group than in the placebo group.
What was the incidence of serious adverse events in the trial?
-Serious adverse events were less common among recipients of semaglutide.
What is the conclusion of the authors regarding semaglutide's effects on patients with type 2 diabetes and high-risk CKD?
-The authors conclude that semaglutide reduced the risk of major kidney disease events among patients with type 2 diabetes and high-risk chronic kidney disease.
Where can the full trial results be found?
-The full trial results are available at NEJM.org.
What is the significance of the 24% risk reduction in major kidney disease events for patients treated with semaglutide?
-The 24% risk reduction is significant as it demonstrates a substantial benefit of semaglutide in reducing the risk of severe kidney complications in patients with type 2 diabetes and CKD.
What does eGFR stand for, and why is it important in this trial?
-eGFR stands for estimated Glomerular Filtration Rate, which is an indicator of kidney function. It is important in this trial as a part of the composite primary outcome measure for major kidney disease events.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

DAPA-CKD: Renal benefits of SGLT2 inhibition in people with and without diabetes | Hiddo Heerspink
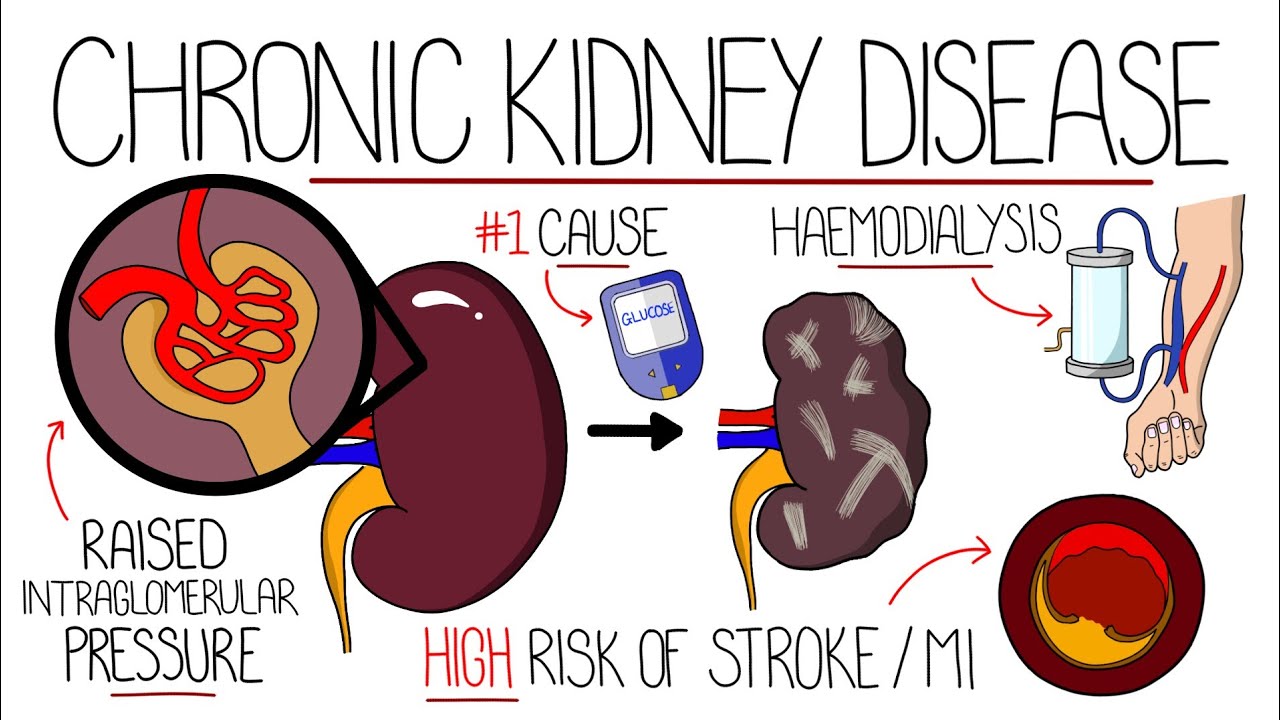
Understanding Chronic Kidney Disease (CKD)

Ozempic Commercial (2025)

INSUFICIÊNCIA RENAL CRÔNICA: SINTOMAS E CAUSAS

Chronic kidney disease: Clinical Nursing Care

Dr. Alok Gupta - 'Low Carb for Renal Patients: My Experience'
5.0 / 5 (0 votes)
