ALKOHOL (ALKANOL) : RUMUS UMUM, JENIS-JENIS ALKANOL, TATA NAMA IUPAC & TRIVIAL, ISOMER
Summary
TLDRThis video explores the chemistry of alcohols (alkanols), discussing their structure, types, and nomenclature. It explains how alcohols are categorized based on the position of the hydroxyl group (primary, secondary, and tertiary alcohols), and also based on the number of hydroxyl groups (monohydric and polyhydric alcohols). The process of naming alcohols according to IUPAC rules is detailed, with examples illustrating the correct procedures for determining names based on structure and functional groups. Additionally, the video covers alcohol isomers, including position isomers, optical isomers, and functional isomers, providing a thorough understanding of alcohol-related compounds.
Takeaways
- 😀 Alcohol is a derivative compound of alkanes containing a hydroxyl group (-OH) with the general formula CnH2n+2O2.
- 😀 Alcohols are classified into three types based on the position of the hydroxyl group: primary, secondary, and tertiary.
- 😀 A primary alcohol has the hydroxyl group attached to a primary carbon, which is a carbon bound to only one other carbon.
- 😀 A secondary alcohol has the hydroxyl group attached to a secondary carbon, which binds to two other carbons.
- 😀 A tertiary alcohol has the hydroxyl group attached to a tertiary carbon, which binds to three other carbons.
- 😀 Alcohols can also be classified based on the number of hydroxyl groups into monoalcohols (one hydroxyl group) and polyalcohols (more than one hydroxyl group).
- 😀 The IUPAC naming system for alcohols includes determining the longest carbon chain (alkyl group) and adding the suffix -ol, e.g., methanol, ethanol, propanol.
- 😀 Polyols are named with the suffix -diol, -triol, etc., depending on the number of hydroxyl groups, e.g., butanediol, propanetriol.
- 😀 In IUPAC nomenclature, numbering of the carbon chain is done to give the hydroxyl group the lowest possible number.
- 😀 In trivial nomenclature, alcohols are named by the alkyl group followed by 'alcohol,' such as ethyl alcohol, propyl alcohol, and isopropyl alcohol.
- 😀 Alcohols can exhibit isomerism, including position isomerism, optical isomerism, and functional isomerism, with examples like 1-propanol and 2-propanol.
Q & A
What are alcohols in chemistry?
-Alcohols are organic compounds derived from alkanes, containing a hydroxyl group (-OH). Their general formula is CnH2n+2O, where 'n' is the number of carbon atoms.
How are alcohols classified based on the position of the hydroxyl group?
-Alcohols are classified into three types based on the position of the hydroxyl group: primary, secondary, and tertiary. In primary alcohols, the hydroxyl group is attached to a primary carbon; in secondary alcohols, it is attached to a secondary carbon; and in tertiary alcohols, it is attached to a tertiary carbon.
What is the structural feature that defines a primary alcohol?
-A primary alcohol has the hydroxyl group attached to a primary carbon atom, which is a carbon that is bonded to only one other carbon atom.
What example of a primary alcohol is mentioned in the script?
-An example of a primary alcohol mentioned is 2-methyl-1-butanol, where the hydroxyl group is attached to the first carbon atom of the butane chain.
How is a secondary alcohol defined?
-A secondary alcohol has the hydroxyl group attached to a secondary carbon, which is a carbon atom bonded to two other carbon atoms.
Can you provide an example of a secondary alcohol from the transcript?
-An example of a secondary alcohol is 2-pentanol, where the hydroxyl group is attached to the second carbon in the pentane chain.
What characterizes a tertiary alcohol?
-A tertiary alcohol has the hydroxyl group attached to a tertiary carbon, which is a carbon atom bonded to three other carbon atoms.
Give an example of a tertiary alcohol from the script.
-An example of a tertiary alcohol is 2-methyl-2-pentanol, where the hydroxyl group is attached to the second carbon, which is bonded to three other carbon atoms.
What are monoalcohols and polyalcohols?
-Monoalcohols are alcohols that contain only one hydroxyl group, while polyalcohols contain more than one hydroxyl group. For example, 1-propanol is a monoalcohol, and 1,2,3-propanetriol (glycerol) is a polyalcohol.
How is the nomenclature of alcohols done according to IUPAC rules?
-The IUPAC nomenclature of alcohols involves determining the longest carbon chain as the parent structure, numbering the chain to give the hydroxyl group the lowest possible number, and naming the compound by adding the suffix 'ol' for monoalcohols or 'diol,' 'triol,' etc., for polyalcohols.
What is the trivial naming system for alcohols?
-In the trivial naming system, alcohols are named by first identifying the alkyl group (e.g., ethyl or propyl) followed by the word 'alcohol.' For example, ethyl alcohol or isopropyl alcohol.
What are the different types of isomerism found in alcohols?
-Alcohols can exhibit three types of isomerism: position isomerism (where the hydroxyl group is attached to different carbon atoms), optical isomerism (due to the presence of an asymmetric carbon atom), and functional isomerism (where alcohols and ethers have the same molecular formula but different functional groups).
Can you explain position isomerism in alcohols?
-Position isomerism in alcohols occurs when two compounds have the same molecular formula but the hydroxyl group is attached to different carbon atoms in the chain. For example, 1-propanol and 2-propanol are position isomers.
What is optical isomerism in alcohols?
-Optical isomerism occurs in alcohols when there is an asymmetric carbon atom, which results in non-superimposable mirror images (enantiomers). An example is 2-butanol, which has one asymmetric carbon.
How does functional isomerism apply to alcohols and ethers?
-Functional isomerism in alcohols and ethers occurs when two compounds have the same molecular formula but differ in their functional groups. For example, propyl alcohol and ethyl methyl ether have the same formula (C3H8O) but different functional groups (hydroxyl group vs. ether group).
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Funções orgânicas oxigenadas - Brasil Escola
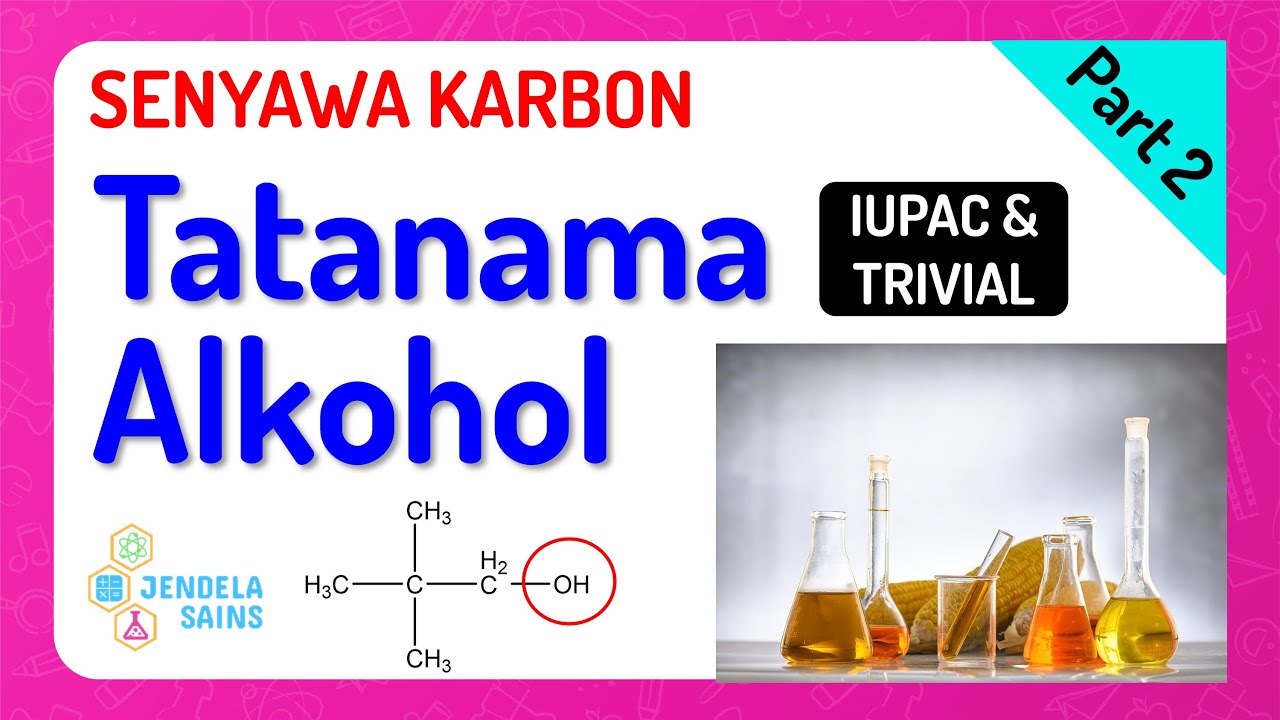
Senyawa Karbon Turunan Alkana • Part 2: Tatanama Alkohol / Alkanol

Tata Nama Senyawa Turunan Alkana | KIMIA KELAS 12

Tests for Alcohols

Alcohols | A-level Chemistry | OCR, AQA, Edexcel

Alcohols | Alcohols, ethers, epoxides, sulfides | Organic chemistry | Khan Academy
5.0 / 5 (0 votes)
