Tests for Alcohols
Summary
TLDRThis video series explores various chemistry experiments, focusing on testing alcohols. It demonstrates methods like the sodium metal test, ester test, silk ammonium nitrate test, and iodoform test, explaining how alcohols react with different substances to identify their types and properties.
Takeaways
- 🧪 Alcohols are organic compounds with a hydroxyl group attached to an alkyl or aryl component.
- 🔍 Alcohols are classified as primary, secondary, or tertiary based on the nature of the alpha carbon.
- 🌟 The sodium metal test is used to identify alcohols by their reaction with sodium to produce sodium alkoxide and hydrogen gas.
- 🍇 The ester test involves reacting alcohols with carboxylic acids in the presence of sulfuric acid to produce sweet-smelling esters.
- 🌈 The ammonium nitrate test uses a reaction with alcohols to form an alkoxy saving complex, which turns blood red in color.
- 💧 The iodoform test checks for alcohols with a specific structure (C-HC-C) that react with iodine and sodium hydroxide to form a yellow precipitate.
- 🔬 Materials needed for the sodium metal test include an organic compound, anhydrous calcium sulfate, sodium metal, test tubes, and other laboratory equipment.
- 🧪 For the ester test, materials include glacial acetic acid, concentrated sulfuric acid, cold water, and test tubes.
- 🌡️ The ammonium nitrate test requires an organic compound, ammonium nitrate reagent, and test tubes.
- 🔍 The iodoform test needs an organic compound, a 1% iodine solution, dilute sodium hydroxide solution, and test tubes.
- 📚 Each test has a specific procedure involving mixing, heating, and observing reactions to identify the presence of alcohols.
Q & A
What is the main focus of the video series?
-The main focus of the video series is to present various experiments in chemistry, specifically demonstrating simple tests for alcohols in this particular video.
What is an alcohol in the context of organic chemistry?
-An alcohol is an organic compound that contains a hydroxyl functional group attached to an alkyl or aryl component, formed by replacing a hydrogen atom of a hydrocarbon with a hydroxyl group.
How are alcohols classified based on the nature of the alpha carbon?
-Alcohols are classified as primary, secondary, or tertiary based on the nature of the alpha carbon, which is the carbon attached to the hydroxyl group.
What is the purpose of the sodium metal test in identifying alcohols?
-The sodium metal test is used to identify alcohols by reacting them with active metals like sodium, which produces sodium alkoxide and the effervescence of hydrogen gas.
What materials are required for the sodium metal test?
-Materials required for the sodium metal test include an organic compound, anhydrous calcium sulfate, sodium metal, test tubes, a funnel, filter papers, a spatula, and forceps.
What is the ester test and what does it involve?
-The ester test involves reacting alcohols with carboxylic acids in the presence of concentrated sulfuric acid to produce sweet-smelling compounds called esters.
What materials are needed for the ester test?
-Materials needed for the ester test include an organic compound, glacial acetic acid, concentrated sulfuric acid, a beaker containing cold water, test tubes, droppers, and a water bath.
What is the Seneca nitrate test and what color does it produce?
-The Seneca nitrate test is a reaction of alcohols with cynic ammonium nitrate, which produces an alkoxy saving for complex that is blood red in color.
What is the purpose of the Iodine test in identifying alcohols?
-The Iodine test is used to identify alcohols with the structure CH3CH2C, which react with iodine in the presence of sodium hydroxide to give sodium carboxylate, reform suleiman, and water, resulting in a yellow precipitate.
What materials are required for the Iodine test?
-Materials required for the Iodine test include an organic compound, a 1% iodine solution, dilute sodium hydroxide solution, test tubes, and droppers.
What is the significance of the water bath in the ester test?
-The water bath in the ester test is used to gently heat the mixture, facilitating the reaction between the alcohol and the carboxylic acid to form esters.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Alcohols | A-level Chemistry | OCR, AQA, Edexcel

Alkohole, wzory, nazwy i właściwości #1 [ Pochodne węglowodorów ]

Práctica de laboratorio: Hidrocarburos
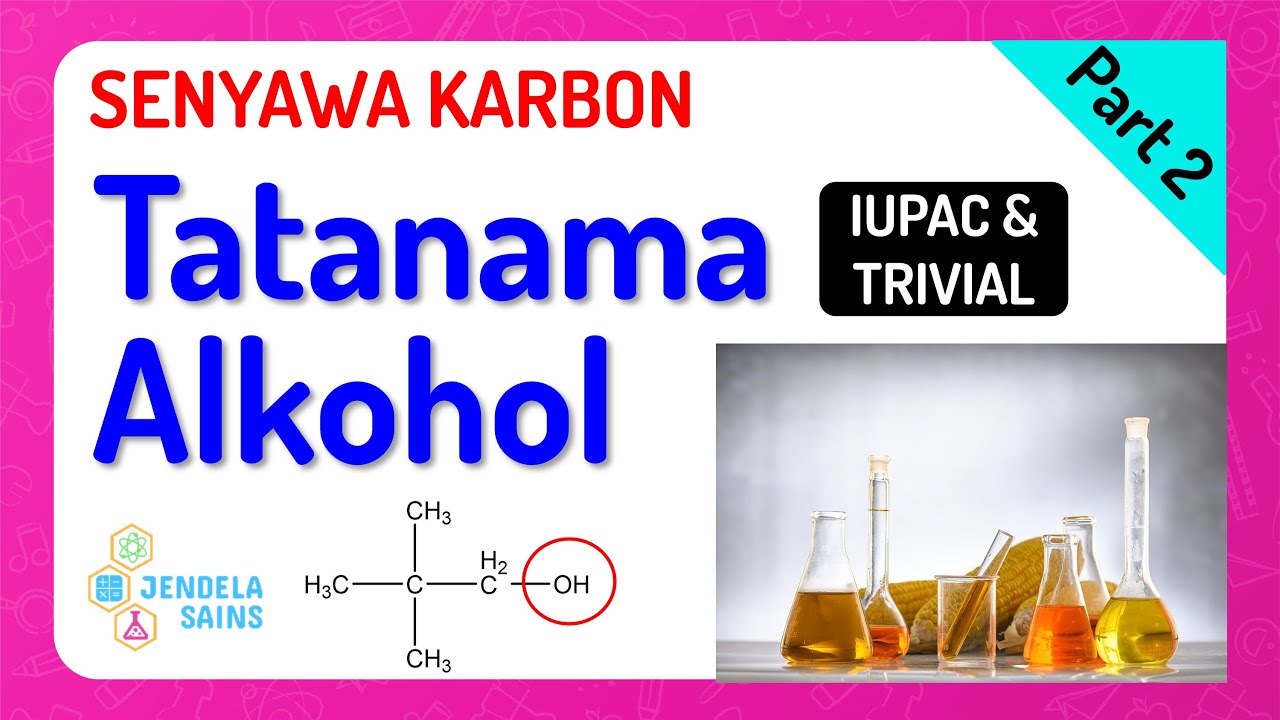
Senyawa Karbon Turunan Alkana • Part 2: Tatanama Alkohol / Alkanol

Keys to Chemistry

Alcohols | Alcohols, ethers, epoxides, sulfides | Organic chemistry | Khan Academy
5.0 / 5 (0 votes)