Termokimia | Sistem & Lingkungan | Reaksi Eksoterm & Endoterm | Entalpi Molar | KIMIA KELAS 11
Summary
TLDRThis video provides an engaging introduction to thermochemistry, exploring the heat changes associated with chemical reactions. It covers essential concepts such as systems (open, closed, and isolated), the distinction between exothermic and endothermic reactions, and the significance of enthalpy changes. Key thermochemical equations are introduced, demonstrating how enthalpy variations relate to chemical processes. The video emphasizes the importance of understanding these principles in chemistry, setting the foundation for further learning in the subject.
Takeaways
- 😀 Thermochemistry studies the heat exchange in chemical reactions.
- 🌍 A **system** is the main focus of study, while the **surroundings** are everything outside the system.
- 🔄 There are three types of systems: **open** (exchanges matter and energy), **closed** (exchanges only energy), and **isolated** (exchanges neither).
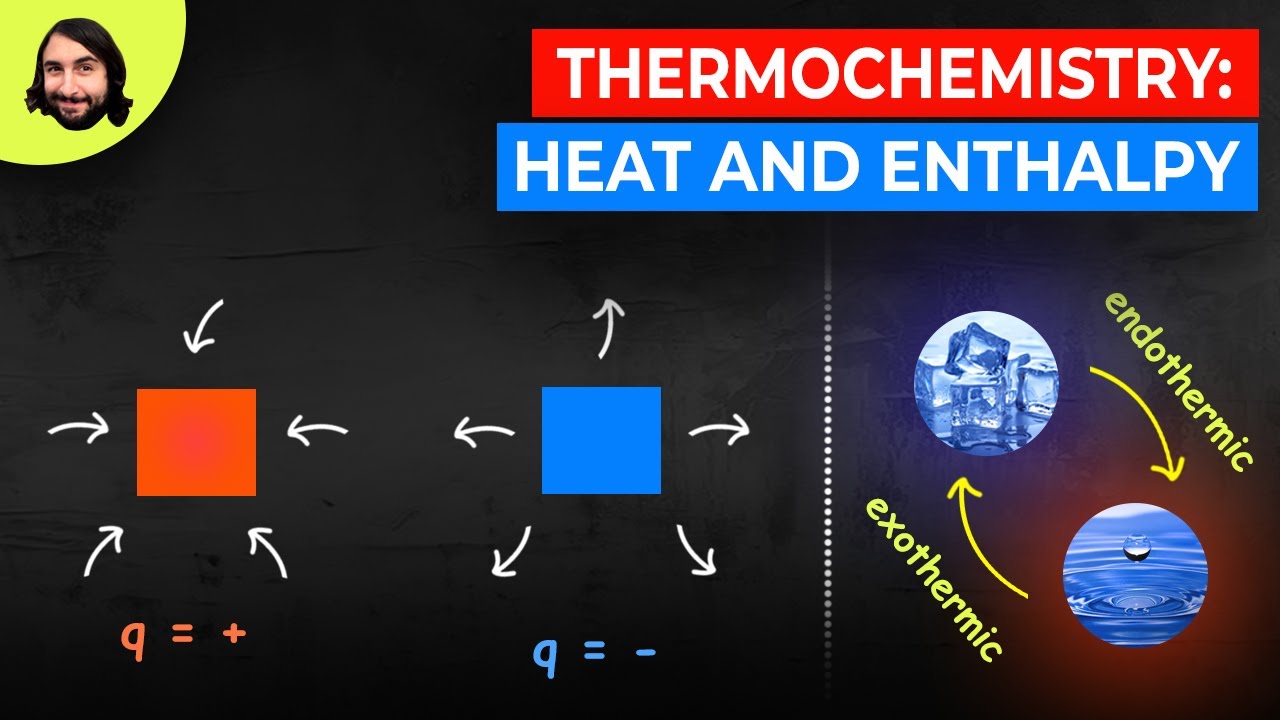
- 🔥 **Exothermic reactions** release heat, indicated by a negative change in enthalpy (ΔH).
- 💧 **Endothermic reactions** absorb heat, indicated by a positive change in enthalpy (ΔH).
- 📉 In exothermic reactions, the products have lower enthalpy than the reactants, while in endothermic reactions, the products have higher enthalpy.
- 📐 **Enthalpy change (ΔH)** is the difference between the enthalpy of products and reactants.
- 🧪 **Thermochemical equations** include the enthalpy change, reflecting whether the reaction is exothermic or endothermic.
- ⚛️ The **standard enthalpy of formation (ΔH°_f)** measures the energy change when one mole of a compound is formed from its elements.
- 💥 The **standard enthalpy of combustion** measures the energy released when one mole of a substance is burned in oxygen.
Q & A
What is thermochemistry?
-Thermochemistry is a branch of chemistry that studies the heat involved in chemical reactions.
What is the difference between a system and its surroundings?
-The system refers to the part of the universe being studied, while the surroundings include everything outside of it.
What are the three types of systems in thermochemistry?
-The three types of systems are open systems (exchange both matter and energy), closed systems (exchange energy but not matter), and isolated systems (exchange neither matter nor energy).
What is an example of an open system?
-An example of an open system is a cup of hot water, where both heat and matter can be exchanged with the environment.
What characterizes an exothermic reaction?
-An exothermic reaction is characterized by the release of heat to the surroundings, resulting in a negative change in enthalpy (ΔH).
How is enthalpy change (ΔH) calculated?
-Enthalpy change (ΔH) is calculated as the difference between the enthalpy of the products and the enthalpy of the reactants: ΔH = Enthalpy of products - Enthalpy of reactants.
What happens to the enthalpy during an endothermic reaction?
-In an endothermic reaction, the system absorbs heat from the surroundings, resulting in a positive change in enthalpy (ΔH).
What is the significance of thermochemical equations?
-Thermochemical equations include the enthalpy change and provide information about the energy involved in the reaction, helping to understand the thermodynamic properties of substances.
What is the standard enthalpy of formation?
-The standard enthalpy of formation (ΔH°f) is the energy change that occurs when one mole of a compound is formed from its elements in their standard states.
How does the enthalpy of a reaction change if the coefficients are multiplied?
-If the coefficients in a thermochemical equation are multiplied, the enthalpy change must also be multiplied by the same factor to maintain the balance of energy in the reaction.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード5.0 / 5 (0 votes)