Termokimia : Sistem dan Lingkungan, Entalpi, Reaksi Eksoterm dan Endoterm
Summary
TLDRIn this engaging session on thermochemistry, the presenter introduces fundamental concepts, including the significance of heat in chemical reactions. Through relatable examples like photosynthesis and campfires, viewers learn about systems and their surroundings, distinguishing between open, closed, and isolated systems. The discussion covers enthalpy and its role in understanding energy changes, emphasizing the differences between exothermic and endothermic reactions. Additionally, practical exercises on writing thermochemical equations enhance comprehension, making this a comprehensive resource for those looking to grasp the essentials of thermochemistry.
Takeaways
- 🌞 Thermochemistry studies the energy changes associated with chemical reactions, including heat transfer.
- 🔍 The system refers to the specific part of the universe being studied, while the surroundings include everything else that can interact with it.
- 🌡️ There are three types of systems: open (mass and energy exchange), closed (energy exchange only), and isolated (no exchange of mass or energy).
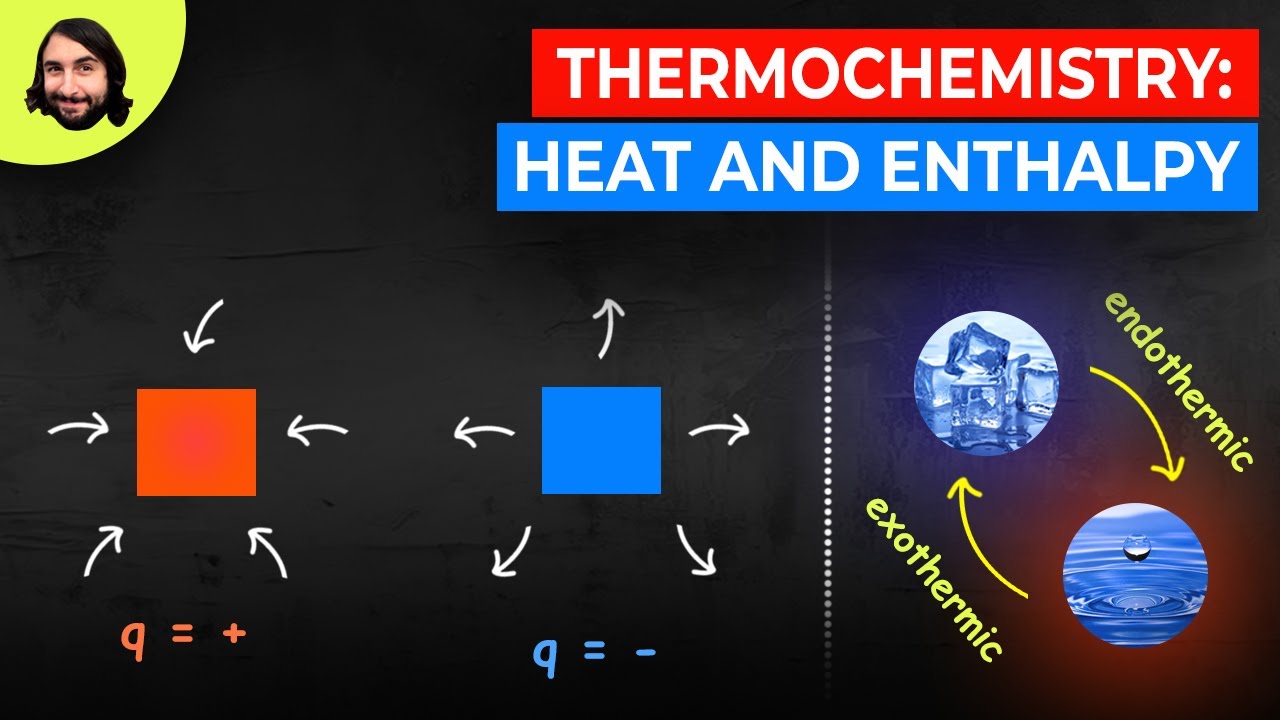
- 🔥 Exothermic reactions release heat, leading to an increase in the temperature of the surroundings, with a negative enthalpy change (ΔH).
- ❄️ Endothermic reactions absorb heat, causing the surroundings to cool down, characterized by a positive enthalpy change (ΔH).
- 📏 Enthalpy (ΔH) is a measure of the total energy in a system, calculated as ΔH = H(products) - H(reactants).
- 🧪 Understanding the distinction between reactants and products is essential for analyzing thermochemical reactions.
- 🌡️ Heat transfer can be observed in everyday situations, such as photosynthesis in trees and warmth from a campfire.
- 📊 Practice problems in thermochemistry often involve writing balanced equations and determining the enthalpy changes for reactions.
- 📚 Knowledge of thermochemistry is crucial for comprehending various scientific principles and real-world applications.
Q & A
What is thermochemistry?
-Thermochemistry is the branch of chemistry that studies the heat energy associated with chemical reactions, particularly the heat absorbed or released during these reactions.
What are some everyday examples of thermochemistry?
-Everyday examples include photosynthesis in plants, which requires energy from sunlight, the warmth felt from a campfire, and the energy changes experienced while riding a motorcycle.
What defines a system in a chemical reaction?
-In a chemical reaction, the system is defined as everything that is being observed or studied, which includes the reactants and products involved in the reaction.
What are the three types of systems described in the script?
-The three types of systems are: 1) Open systems, which allow for the transfer of both mass and energy; 2) Closed systems, which allow for energy transfer but not mass transfer; and 3) Isolated systems, which do not allow for the transfer of either mass or energy.
What is enthalpy and how is it symbolized?
-Enthalpy is a measure of the total energy of a thermodynamic system and is symbolized by 'H'. The change in enthalpy is represented by 'ΔH'.
What is the difference between exothermic and endothermic reactions?
-Exothermic reactions release heat into the surroundings, causing the environment to feel warmer, while endothermic reactions absorb heat from the surroundings, making the environment feel cooler.
What happens to the temperature of the surroundings during an exothermic reaction?
-During an exothermic reaction, the temperature of the surroundings increases due to the release of heat energy from the system.
What is the significance of the sign of ΔH in thermochemical reactions?
-The sign of ΔH indicates whether the reaction is exothermic or endothermic. A negative ΔH value indicates an exothermic reaction (heat released), while a positive ΔH value indicates an endothermic reaction (heat absorbed).
How do you write a thermochemical equation for the formation of water?
-For the formation of one mole of water from hydrogen and oxygen, the equation is: 2H2(g) + O2(g) → 2H2O(l), with ΔH = -571 kJ.
What is the relationship between calorimetry and thermochemistry?
-Calorimetry is the experimental technique used to measure the heat absorbed or released during chemical reactions, which is essential for understanding thermochemical processes.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
5.0 / 5 (0 votes)