Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems
Summary
TLDRThis educational video script delves into the intricacies of orbital diagrams, guiding viewers through the process of drawing and populating orbitals with electrons for elements like nitrogen and magnesium. It explains the concept of electron configuration, emphasizing the importance of filling orbitals in order of increasing energy and the rule of Hund's to fill degenerate orbitals with parallel spins. The script distinguishes between paramagnetic and diamagnetic substances, providing examples with nitrogen and magnesium. It also addresses the complexities of transition metal ions, cautioning against common mistakes in electron subtraction. The aim is to equip viewers with a clear understanding of orbital diagrams and their significance in chemistry.
Takeaways
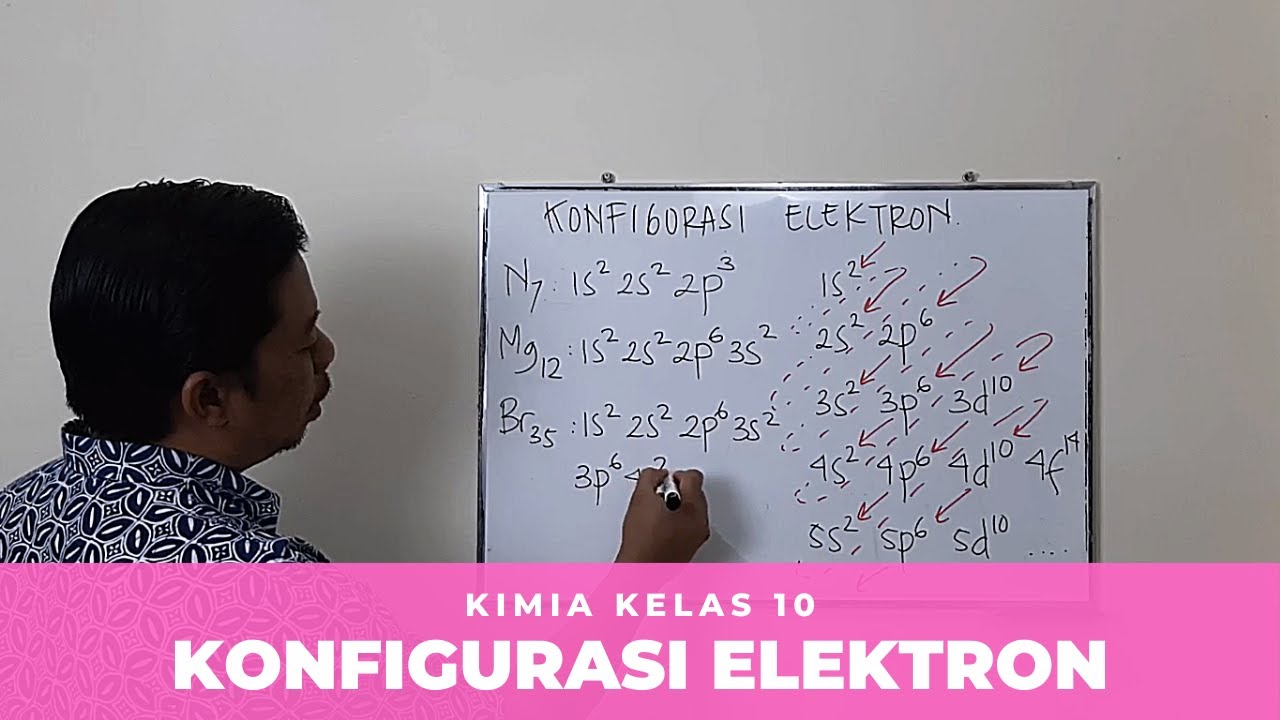
- 🔬 Nitrogen's atomic number is 7, and its electron configuration is 1s2 2s2 2p3, with three unpaired electrons making it paramagnetic.
- 📝 To draw an orbital diagram, first determine the electron configuration, then fill the orbitals from lowest to highest energy, following Hund's rule for degenerate orbitals.
- 🧲 Paramagnetic substances have unpaired electrons and are weakly attracted to a magnetic field, while diamagnetic substances have paired electrons and are weakly repelled.
- 🌟 Magnesium, with an atomic number of 12, has a filled 1s, 2s, and 2p orbitals, and a partially filled 3s orbital, making it diamagnetic.
- 💡 You don't always need to write out the electron configuration; you can directly fill the orbital diagram by counting electrons.
- 🌐 Phosphorus, with 15 electrons, will have a completely filled 3p sublevel, similar to magnesium but with an extra electron pair in the 3s orbital.
- ⚛️ For ions, determine the total number of electrons by adding or subtracting the charge from the atomic number, then fill the orbitals accordingly.
- 🔵 The sulfide ion (S^2-) has 18 electrons, which when filled in the orbital diagram, results in a diamagnetic ion with all paired electrons.
- ⚜️ For transition metal ions like aluminum (Al^3+), subtract electrons from the highest energy level first, not just from the total count.
- 🔶 With transition metals, especially ions, write the electron configuration of the neutral atom first, then adjust for the ion's charge by removing electrons from the highest energy levels.
Q & A
What is the atomic number of nitrogen?
-The atomic number of nitrogen is 7.
What is the electron configuration of nitrogen?
-The electron configuration of nitrogen is 1s2 2s2 2p3.
How many unpaired electrons does nitrogen have?
-Nitrogen has three unpaired electrons.
Is nitrogen paramagnetic or diamagnetic?
-Nitrogen is paramagnetic due to the presence of unpaired electrons.
What is the significance of degenerate orbitals in filling electron diagrams?
-Degenerate orbitals have the same energy, and electrons are filled one at a time with parallel spins in each orbital.
Why is it not necessary to write the electron configuration before drawing the orbital diagram?
-It is not necessary to write the electron configuration before drawing the orbital diagram because you can directly fill the orbitals with electrons until you reach the correct total number for the atom or ion.
What is the electron configuration of magnesium?
-The electron configuration of magnesium is 1s2 2s2 2p6 3s2.
Is magnesium paramagnetic or diamagnetic?
-Magnesium is diamagnetic because it contains only paired electrons.
How many electrons does the sulfide ion have and why?
-The sulfide ion has 18 electrons because it is a sulfur atom with an additional two electrons due to its -2 charge.
Is the sulfide ion paramagnetic or diamagnetic?
-The sulfide ion is diamagnetic as it contains only paired electrons.
How do you determine the number of electrons in a transition metal ion like cobalt plus 2?
-For transition metal ions like cobalt plus 2, you first write the electron configuration of the neutral atom, then remove electrons from the highest energy level to account for the charge. For cobalt plus 2, you would remove two electrons from the 4s orbital, resulting in a configuration of 4s0 3d7.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

3.6.6 - Distribuição eletrônica completa dos elétrons do oxigênio magnésio, titânio e estanho

An Introduction to Inorganic Chemistry- Lecture 2

A Level Chemistry Revision "Electron Configuration"
Konfigurasi Elektron | KIMIA KELAS 10

Hund's Rule

Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle - Electron Configuration - Chemistry
5.0 / 5 (0 votes)
