Osmosis | #aumsum #kids #science #education #children
Summary
TLDRThis video explains the concept of osmosis through the example of raisins. It demonstrates how water moves through a selectively permeable membrane, from areas of high water concentration to low. The raisins absorb water when placed in a hypotonic solution (water), causing them to swell. When placed in a hypertonic solution (sugar syrup), water moves out of the raisins, leading them to shrink. The video provides a clear, simple explanation of osmosis and the different types of solutions affecting it.
Takeaways
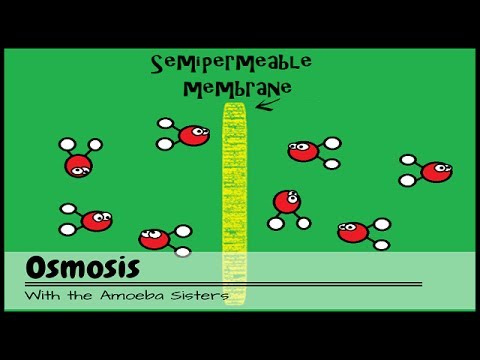
- 😀 Osmosis is the movement of water molecules through a selectively permeable membrane.
- 😀 Water moves from regions of high water concentration to regions of low water concentration.
- 😀 A practical example to understand osmosis is using raisins in water.
- 😀 When raisins are placed in water, they swell because they absorb water.
- 😀 Before the raisins are placed in water, they have low water concentration inside them.
- 😀 After absorbing water, the raisins swell due to higher external water concentration.
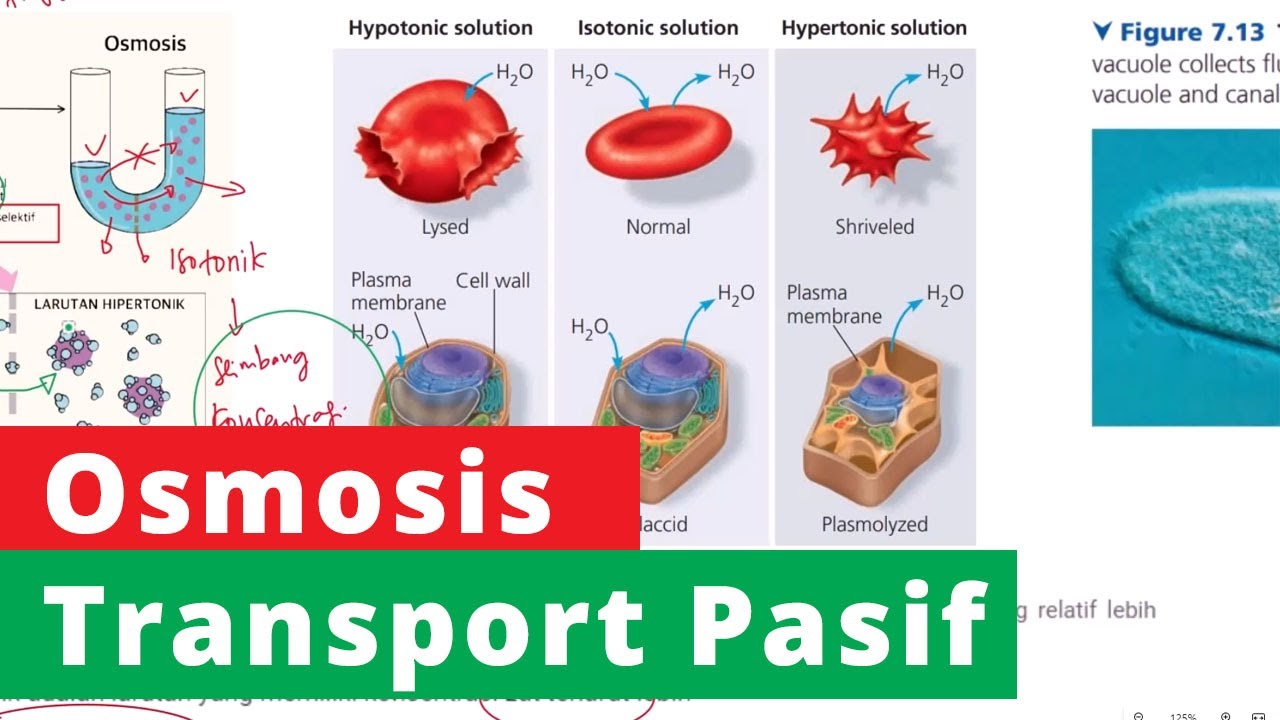
- 😀 A solution with higher water concentration outside the cell is called a hypotonic solution.
- 😀 The person doesn’t like raisins, but the experiment still serves to explain osmosis.
- 😀 When raisins are placed in sugar syrup, the water inside them moves out.
- 😀 In sugar syrup, there is lower water concentration compared to the raisins, causing water to leave the raisins.
- 😀 A solution with lower water concentration outside the cell is called a hypertonic solution.
Q & A
What is osmosis?
-Osmosis is the movement of water molecules through a selectively permeable membrane from a region of high water concentration to a region of low water concentration.
What happens to raisins when they are placed in water?
-When raisins are placed in water, they swell, indicating that they have absorbed water.
Why do raisins swell when placed in water?
-Raisins swell because the water molecules move from the area of higher water concentration outside the raisin to the area of lower water concentration inside the raisin, causing them to absorb water.
What is a hypotonic solution?
-A hypotonic solution is one where there is a higher concentration of water outside the cell compared to the inside, leading to water moving into the cell.
What happens when raisins are placed in a sugar syrup?
-When raisins are placed in a sugar syrup, the water inside the raisins moves out to the syrup because the syrup has a lower water concentration compared to the inside of the raisins.
Why do raisins shrink when placed in sugar syrup?
-Raisins shrink in sugar syrup because the concentration of water inside the raisins is higher than in the syrup. This causes water to leave the raisins, leading to a loss of volume.
What is a hypertonic solution?
-A hypertonic solution is one where there is a lower concentration of water outside the cell compared to inside, causing water to move out of the cell.
What is the significance of water concentration in osmosis?
-The concentration of water plays a crucial role in osmosis, as water moves from areas of higher water concentration to areas of lower water concentration to balance the concentration levels across a membrane.
How does osmosis affect the size of cells or objects placed in different solutions?
-Osmosis can cause cells or objects to swell when placed in a hypotonic solution (higher water concentration outside) and shrink when placed in a hypertonic solution (lower water concentration outside).
What is the purpose of using raisins to demonstrate osmosis?
-Raisins are used to demonstrate osmosis because they absorb water when placed in a hypotonic solution (water) and lose water when placed in a hypertonic solution (sugar syrup), providing a clear visual representation of osmotic processes.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes
5.0 / 5 (0 votes)