Gases - Parte 2
Summary
TLDRProfessor Rodrigo Cordeiro's lecture explores the behavior of gases, focusing on the ideal gas law (Pv = nRT) and its limitations. The ideal gas law, despite its successes, doesn't account for intermolecular interactions, which are significant at high pressures and low temperatures. The lecture covers the application of the ideal gas law to gas mixtures and the kinetic theory of gases. It also introduces the van der Waals equation, which corrects for deviations from ideal behavior. The lecture emphasizes the need to understand both ideal and real gas behaviors in various practical situations.
Takeaways
- 😀 The ideal gas law is expressed as Pv = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin.
- 😀 The ideal gas law is based on the assumption that gas molecules don't interact with each other and are far apart, making it a good approximation under normal conditions.
- 😀 The ideal gas equation is applied universally to all gases, regardless of their chemical identity, because intermolecular forces are assumed to be negligible.
- 😀 The ideal gas constant (R) has a value of 8.314 J/(mol·K) or 0.0821 L·atm/(mol·K) depending on the units used.
- 😀 The ideal gas equation is commonly used to calculate volume, pressure, and temperature of gases under standard conditions, such as STP (standard temperature and pressure).
- 😀 The behavior of gas molecules is described by the kinetic theory, which relates temperature to the average kinetic energy of molecules.
- 😀 The average speed of gas molecules increases with temperature, and the distribution of molecular speeds follows a pattern with most molecules having intermediate speeds.
- 😀 The van der Waals equation accounts for deviations from ideal behavior by modifying the ideal gas equation to account for intermolecular forces and the finite volume of molecules.
- 😀 At high pressures, intermolecular interactions become significant, causing gases to deviate from ideal behavior, and at low temperatures, molecular interactions also become more important.
- 😀 The van der Waals equation includes two constants, 'a' and 'b', which account for attractive forces and the volume occupied by gas molecules, respectively.
- 😀 The ideal gas law is most accurate at low pressures and high temperatures, while the van der Waals equation is used when gases are under high pressures or low temperatures, though even this equation has limitations.
Q & A
What is the ideal gas equation and how is it derived?
-The ideal gas equation is Pv = nRT. It is derived from the relationships between pressure, volume, temperature, and the number of moles of gas, where pressure is directly proportional to temperature and the number of moles, and volume is inversely proportional to pressure. A constant, R (the ideal gas constant), is used to unify these relationships.
Why does the ideal gas equation not distinguish between different types of gases?
-The ideal gas equation assumes that gas molecules are very far apart, making intermolecular forces negligible. As a result, all gases are assumed to behave the same way regardless of their chemical identity, meaning the equation applies universally.
How does the ideal gas equation apply to gas mixtures?
-For gas mixtures, the ideal gas equation applies to each individual component. The total pressure of the mixture is the sum of the partial pressures of each component. The equation treats each gas independently since the molecules are assumed to behave separately.
What is the kinetic theory of gases, and how does it relate to temperature?
-The kinetic theory of gases describes gas behavior based on the random motion of molecules. It states that the temperature of a gas is related to the average kinetic energy of its molecules. As the temperature increases, the molecules move faster and their average kinetic energy increases.
What happens to the speed distribution of gas molecules when the temperature is increased?
-When the temperature increases, the average speed of gas molecules increases. The distribution of speeds becomes broader and shifted toward higher speeds, though there are still some very slow and very fast molecules.
How does the average speed of molecules vary between different gases at the same temperature?
-At the same temperature, lighter molecules, like helium, have a higher average speed than heavier molecules, like nitrogen, because their kinetic energy is the same but their mass is smaller, which results in a higher velocity.
Under what conditions do gases deviate from ideal behavior?
-Gases deviate from ideal behavior at high pressures and low temperatures. At high pressures, molecules are closer together, and intermolecular forces become significant. At low temperatures, intermolecular attractions are stronger and more noticeable, leading to deviations from ideality.
What is the van der Waals equation, and how does it modify the ideal gas law?
-The van der Waals equation modifies the ideal gas law to account for deviations in gas behavior at high pressures and low temperatures. It introduces two constants, 'a' and 'b', to correct for intermolecular interactions and the finite volume of gas molecules.
Why is the van der Waals equation not always sufficient to describe gas behavior?
-While the van der Waals equation accounts for intermolecular forces and molecular volume, it may still fail in extreme conditions, such as very high pressures or very low temperatures, where more complex, empirical equations may be required.
How does the concept of intermolecular forces affect the pressure in a gas?
-When intermolecular forces are attractive, they cause gas molecules to move closer together, reducing the pressure exerted on the walls of the container. The van der Waals equation accounts for this by subtracting a constant factor (a) from the pressure, reflecting the effect of these interactions.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

Gas Ideal dan Gas Nyata - Kimia Fisika
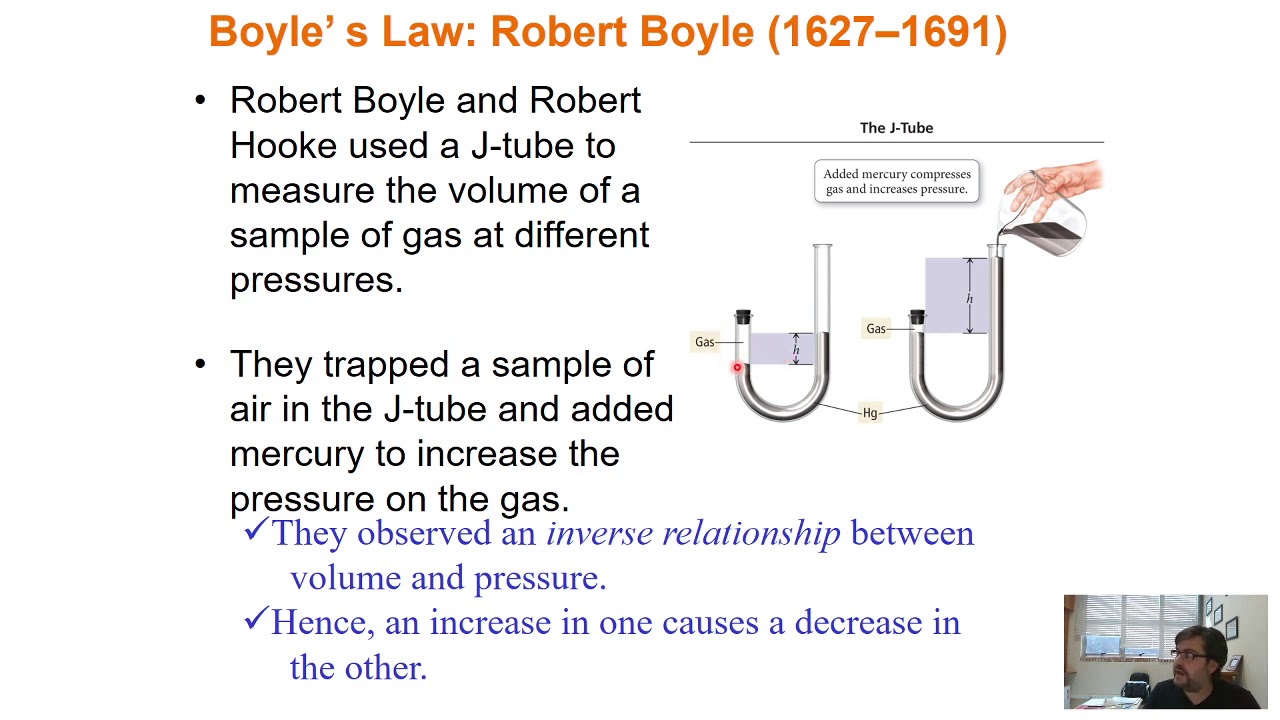
Curso de Físico-Química - Gases Parte 1: Idealidade, Pressão, Lei de Boyle

Transformações gasosas, vamos entender?

Teori Kinetik Gas | Contoh Soal Materi Teori Kinetik Gas | Fisika SMA

PERSAMAAN GAS IDEAL | Teori Kinetik Gas dan Termodinamika #2 - Fisika Kelas 11

The Ideal Gas Law: Crash Course Chemistry #12
5.0 / 5 (0 votes)
