Analisis Kualitatif & Kuantitatif Bahan Baku & Tablet Alopurinol secara Spektrofotometri UV Visible
Summary
TLDRThis video provides a comprehensive guide on qualitative and quantitative analysis of allopurinol raw materials and tablets using UV-Visible spectrophotometry with a calibration curve technique. It outlines the preparation process, including the use of sodium hydroxide as a solvent, and details the steps for creating calibration curves, measuring absorbance, and calculating the concentration of allopurinol. The video emphasizes both qualitative analysis, based on spectral similarity, and quantitative analysis, including calculating the purity of allopurinol in raw materials and tablets. The video concludes by evaluating whether the allopurinol meets the standards set by the Indonesian Pharmacopoeia.
Takeaways
- 😀 Spectrophotometry UV-visible is used for both qualitative and quantitative analysis of compounds like allopurinol, which contains conjugated double bonds and atoms with free electron pairs.
- 😀 The method can analyze both pure compounds and mixtures, as long as interfering substances can either be accounted for or do not affect the analysis.
- 😀 Key materials for the analysis include allopurinol reference substance, allopurinol tablet samples, sodium hydroxide as a solvent, and UV-visible spectrophotometer.
- 😀 The process begins with preparing a calibration curve by selecting a solvent based on the Indonesian Pharmacopoeia, and finding the molar absorptivity of allopurinol.
- 😀 The molar absorptivity of allopurinol is 7090 L/mol·cm, and concentration predictions are made to obtain absorbance values around 0.2 using Beer’s Law.
- 😀 A reference stock solution of allopurinol (1000 µg/mL) is prepared, and then diluted to obtain six concentrations, which are measured for absorbance at the maximum wavelength.
- 😀 The maximum wavelength for allopurinol is determined to be 256 nm, and absorbance is measured across various concentrations to generate a calibration curve.
- 😀 The calibration curve's regression equation (y = 0.05527x + 0.0223) is used for quantitative analysis.
- 😀 To analyze the allopurinol content in tablets, tablet samples are processed by grinding and dissolving in sodium hydroxide, followed by ultrasonic treatment and dilution.
- 😀 After preparation, the tablet solution is analyzed, and the concentration of allopurinol is calculated using the calibration curve, with results compared to the required pharmacopoeia standards.
Q & A
What is the main technique used for analyzing allopurinol in the video?
-The main technique used is UV-Visible spectrophotometry with the calibration curve method.
What is the general purpose of UV-Visible spectrophotometry?
-UV-Visible spectrophotometry is used for both qualitative and quantitative analysis of compounds with conjugated double bonds and atoms with free electron pairs, such as allopurinol.
What is the molar absorptivity value for allopurinol mentioned in the transcript?
-The molar absorptivity value for allopurinol is 7090 L/mol·cm.
What solvent is used in the analysis of allopurinol in the video?
-The solvent used is 0.1 N sodium hydroxide (NaOH).
How is the calibration curve created for the analysis?
-The calibration curve is created by preparing six variations of allopurinol concentrations and measuring their absorbance at the maximum wavelength of 256 nm using UV-Visible spectrophotometry.
What is the equation for the regression line obtained from the calibration curve?
-The equation for the regression line is y = 0.05527x + 0.0223, with a correlation coefficient of 0.998.
What is the purpose of measuring the maximum wavelength of allopurinol?
-Measuring the maximum wavelength allows for the determination of the optimal wavelength for absorbance measurement, which in this case is 256 nm for pure allopurinol.
What procedure is followed when preparing the allopurinol sample for spectrophotometric analysis?
-The sample is prepared by dissolving 100 mg of pure allopurinol in a 100 ml volumetric flask with NaOH, sonicating the solution, and performing dilution steps to ensure accurate concentration measurements.
How is the purity of the allopurinol sample determined?
-The purity of the allopurinol sample is determined by interpolating the absorbance values into the regression equation and calculating the percentage purity based on the molar absorptivity and concentration.
What should be done if the allopurinol tablet's spectrum is slightly different from the standard?
-If the tablet's spectrum is slightly different, but still similar to the standard, it is considered to have the same qualitative characteristics as pure allopurinol, indicating that the tablet contains the active ingredient.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

Spektrofotometri UV-Vis Simple Simultaneous Equation (SSE): Kadar Parasetamol & Kofein dalam Tablet
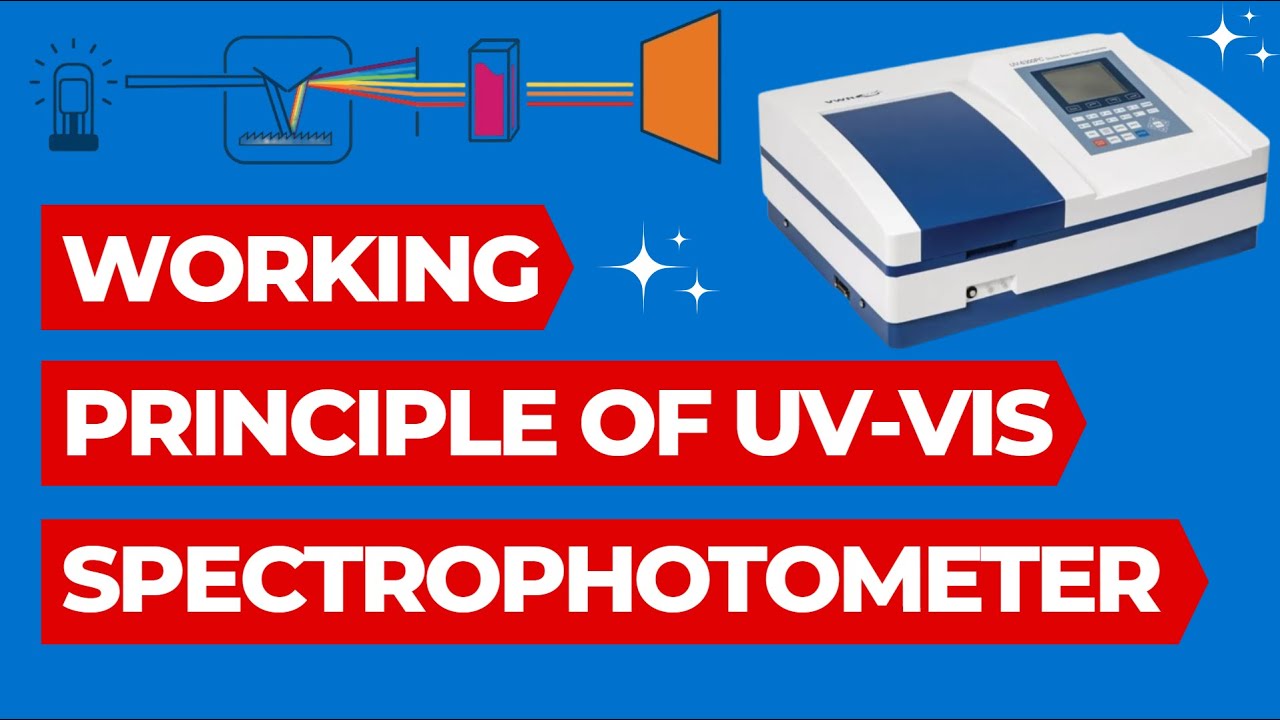
Working Principle of UV Spectrophotometer

A n I 9 Spektrofotometer

Prak. Biokimia Uji Kualitatif dan Kuantitatif Karbohidrat 1

Spektrofotometer UV Vis Double Beam - Pengertian, Fungsi, Jenis-Jenis dan Bagian-Bagian

¿Qué son las TÉCNICAS DE INVESTIGACIÓN? Tipos, características y ejemplos
5.0 / 5 (0 votes)
