Gibbs Energy and equilibrium constants
Summary
TLDRThis video explains the relationship between the equilibrium constant (K) and the standard Gibbs energy change (ΔG) in thermodynamics. It highlights the significance of the natural logarithm of K in Equation 15.4, where a more positive K corresponds to a more negative ΔG. The derivation of this equation is also discussed, beginning with the basic concept of Gibbs energy, using the activity of reactants and products, and simplifying to the final expression. The key points include the connection between equilibrium conditions, where ΔG is zero, and how this leads to the equilibrium constant expression.
Takeaways
- 😀 Equation 15.4 is a fundamental equation in thermodynamics, linking the equilibrium constant (K) to the standard Gibbs free energy change (ΔG).
- 😀 The equation uses the natural logarithm of K, which is crucial for understanding the relationship between Gibbs energy and the equilibrium state.
- 😀 The negative sign in the equation indicates that as K becomes more positive, ΔG standard becomes more negative, showing a more spontaneous reaction.
- 😀 A highly spontaneous reaction has a large positive value for K and a large negative value for ΔG.
- 😀 The derivation of Equation 15.4 starts with the simplest possible reaction where one mole of reactant produces one mole of product.
- 😀 The Gibbs free energy of a substance is given by its standard Gibbs energy plus RT times the logarithm of the activity of the substance.
- 😀 Activity refers to the concentration or partial pressure of a substance relative to its standard concentration or pressure.
- 😀 The equation for Gibbs energy changes is derived by substituting expressions for G products and G reactants into the formula for ΔG.
- 😀 The expression for ΔG can be simplified into an equation involving the activities of the reactants and products.
- 😀 At equilibrium, ΔG is zero and the ratio of the activities (products/reactants) equals the equilibrium constant K, leading to the final form of Equation 15.4.
- 😀 Equation 15.4 can be written as ΔG standard = -RT ln K, which shows the relationship between Gibbs free energy change and the equilibrium constant.
Q & A
What does Equation 15.4 link together in thermodynamics?
-Equation 15.4 links the equilibrium constant (K) to the standard Gibbs energy change (ΔG°).
What is the significance of the negative sign in Equation 15.4?
-The negative sign indicates that as the equilibrium constant (K) increases (becomes more positive), the standard Gibbs energy change (ΔG°) becomes more negative, which corresponds to a more spontaneous reaction.
How does the equilibrium constant (K) relate to reaction spontaneity?
-A large positive value for K indicates a reaction that is highly spontaneous, which corresponds to a large negative value for ΔG°. This shows that the reaction tends to proceed towards products at equilibrium.
What does the equation ΔG = ΔG° + RT ln(a_product/a_reactant) represent?
-This equation represents the Gibbs energy change (ΔG) for a reaction at any point, not necessarily at equilibrium. It shows the relationship between the Gibbs energy change, the standard Gibbs energy change, and the activities of the reactants and products.
How are activities of reactants and products defined in the context of this equation?
-For gases, the activity is the partial pressure of the gas divided by the standard pressure. For solutions, the activity is the concentration of the solute divided by the standard concentration.
What happens when a reaction reaches equilibrium in terms of Gibbs energy?
-At equilibrium, the Gibbs energy change (ΔG) for the reaction becomes zero, as the system has reached a state of minimum free energy.
How does Equation 15.4 relate to the equilibrium constant K?
-At equilibrium, the ratio of product to reactant activities equals the equilibrium constant (K). This leads to the equation ΔG° = -RT ln(K), which connects the standard Gibbs energy change to K.
What is the significance of ΔG° being negative or positive?
-A negative value for ΔG° indicates a spontaneous reaction, while a positive value for ΔG° indicates a non-spontaneous reaction. The magnitude of ΔG° also reflects the driving force of the reaction.
What is the role of temperature (T) in Equation 15.4?
-The temperature (T) is a factor in the equation ΔG° = -RT ln(K), as it directly affects the value of ΔG° through the product RT, influencing the spontaneity of the reaction.
Why is the natural logarithm (ln) used in these thermodynamic equations?
-The natural logarithm is used because it simplifies the relationship between the equilibrium constant and the Gibbs energy change. It ensures that the equation holds under the principles of thermodynamics and that the units remain consistent.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes
Thermodynamics and Energy Diagrams: Crash Course Organic Chemistry #15

05. MG2112 Termodinamika Metalurgi (S01: Kesetimbangan, Energi Bebas Gibbs, Potensial Kimia)
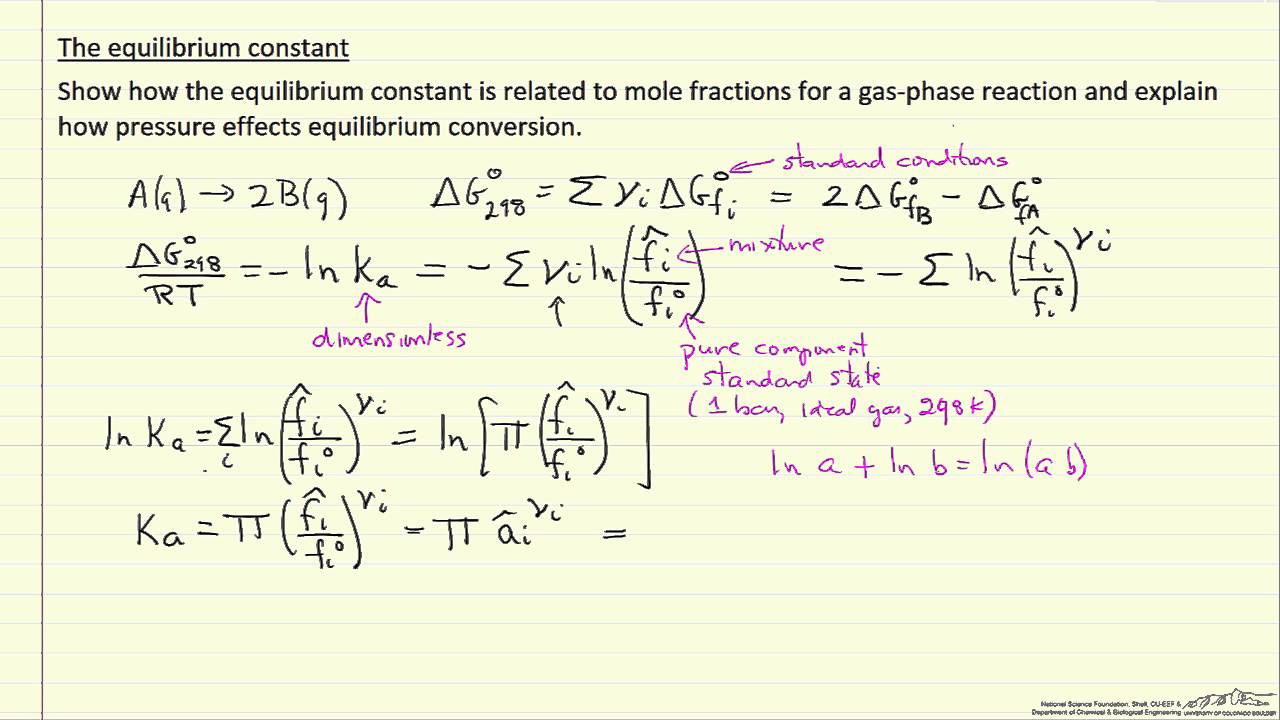
The Equilibrium Constant
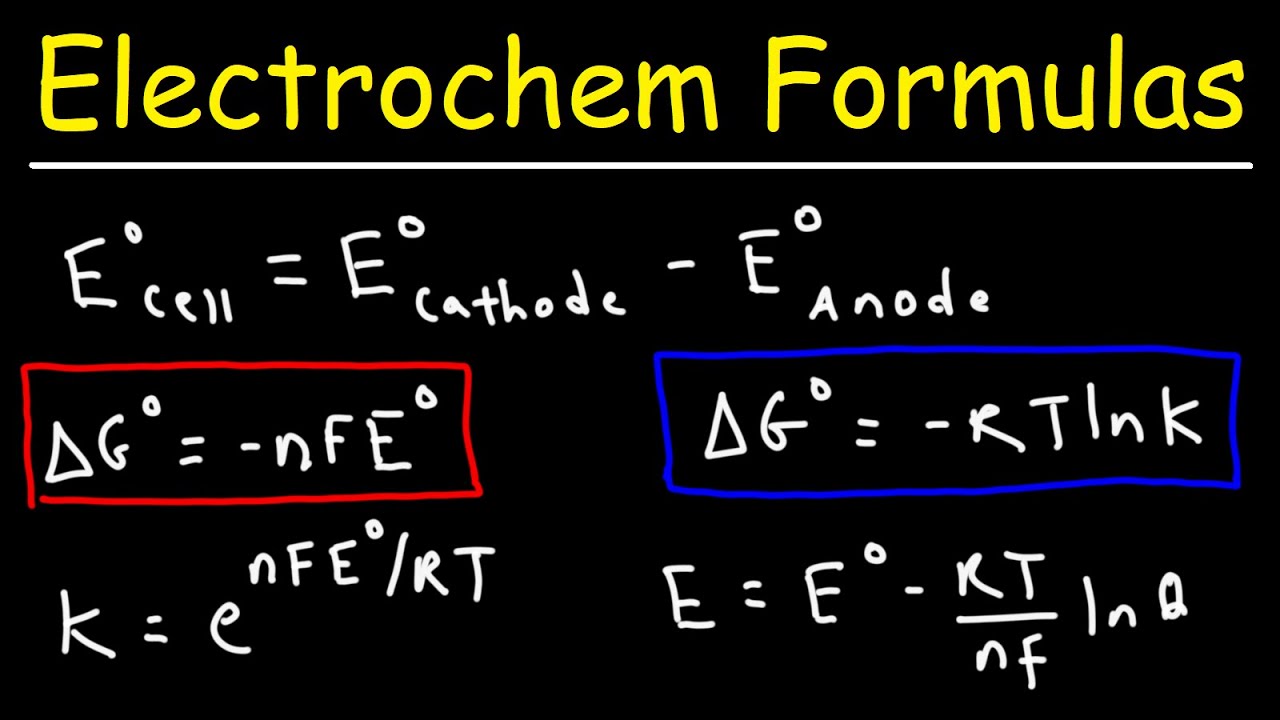
Electrochemistry Formulas - Gibbs Free Energy, Equilibrium K, Cell Potential, Nernst Equation

18.7 Cell Potential and Free Energy in Galvanic Cells
Using Gibbs Free Energy
5.0 / 5 (0 votes)
