Termokimia (3) | Jenis - Jenis Perubahan Entalpi Standar
Summary
TLDRIn this educational video, the presenter discusses standard enthalpy changes, specifically focusing on various types such as enthalpy of formation, decomposition, combustion, neutralization, atomization, fusion, sublimation, and vaporization. Each type is explained with definitions and examples, highlighting the caloric changes involved during chemical reactions under standard conditions. The video aims to enhance understanding of thermochemistry concepts, emphasizing the importance of enthalpy in chemical processes. It provides clear illustrations and practical examples to help students grasp the material effectively.
Takeaways
- 😀 Standard enthalpy change (ΔH) is measured under standard conditions of 1 ATM pressure and 25°C temperature.
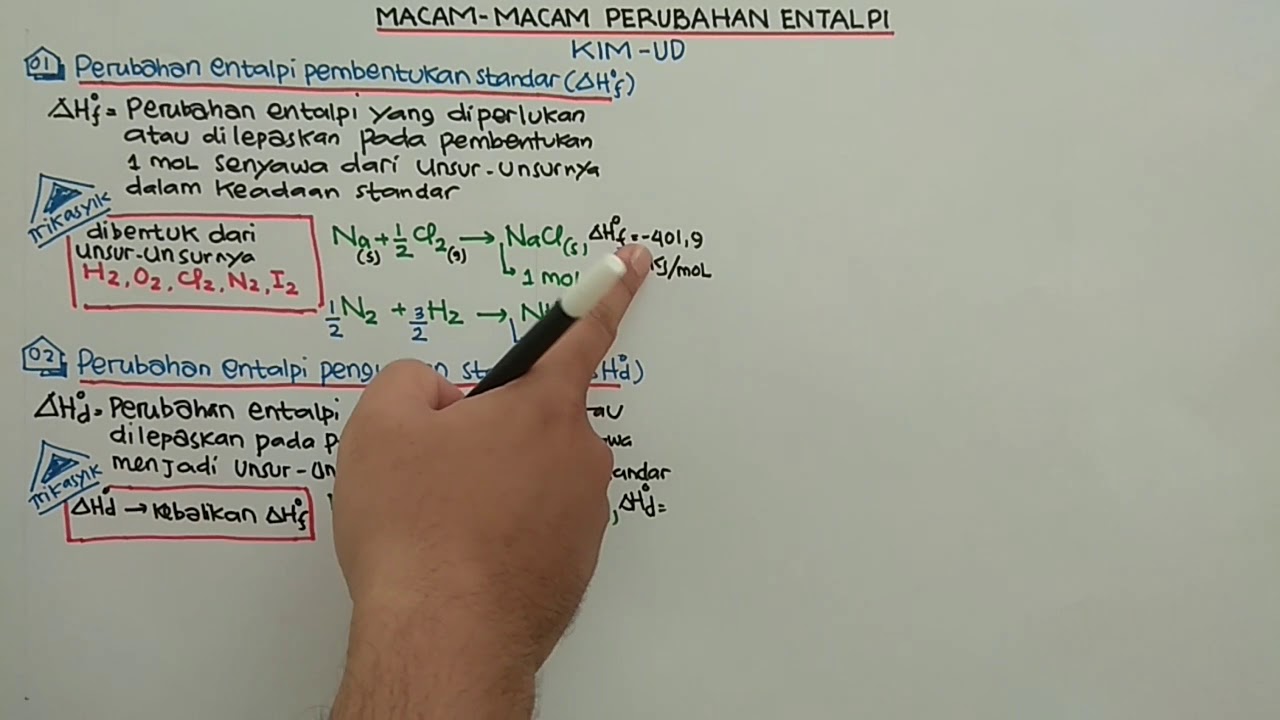
- 😀 The standard enthalpy of formation (ΔHf) is the heat change when one mole of a compound is formed from its elements in their standard states.
- 😀 ΔHf can be either negative (exothermic reaction) or positive (endothermic reaction).
- 😀 Coefficients in the chemical equation for ΔHf must equal one, indicating the formation of one mole of product.
- 😀 The standard enthalpy of decomposition (ΔHd) is the heat change required to break one mole of a compound into its elements.
- 😀 ΔHd is the reverse of ΔHf; if ΔHf is negative, ΔHd will be positive.
- 😀 The standard enthalpy of combustion (ΔHc) is the heat released during the complete combustion of one mole of a substance with oxygen.
- 😀 ΔHc is always negative since combustion reactions are exothermic.
- 😀 The enthalpy of neutralization (ΔHn) refers to the heat change during the neutralization of one mole of acid by a base.
- 😀 The enthalpy of phase changes, such as fusion (melting) and vaporization, indicates the heat absorbed or released when a substance transitions between solid, liquid, and gas phases.
Q & A
What is the definition of standard enthalpy change?
-Standard enthalpy change (ΔH) is the heat change measured under standard conditions, specifically at a pressure of 1 ATM and a temperature of 25°C.
What does the standard enthalpy of formation (ΔH_f) represent?
-The standard enthalpy of formation (ΔH_f) represents the heat change that occurs when one mole of a compound is formed from its elements in their standard states.
Can the value of ΔH_f be negative, and what does it imply?
-Yes, ΔH_f can be negative, indicating that the formation reaction is exothermic and releases heat.
What is the significance of the coefficient '1' in ΔH_f?
-The coefficient '1' indicates that the enthalpy change is measured for the formation of one mole of the compound.
How does the standard enthalpy of decomposition (ΔH_d) relate to ΔH_f?
-The standard enthalpy of decomposition (ΔH_d) is the reverse of ΔH_f; if ΔH_f is negative (exothermic), then ΔH_d will be positive (endothermic) and vice versa.
What is the characteristic of the standard enthalpy of combustion (ΔH_c)?
-The standard enthalpy of combustion (ΔH_c) is always negative because it represents the heat released during the complete combustion of one mole of a substance in oxygen.
What type of reaction does the standard enthalpy of neutralization (ΔH_n) involve?
-ΔH_n involves the reaction of one mole of an acid with one mole of a base, resulting in the formation of salt and water, and it is an exothermic reaction.
What does the standard enthalpy of atomization (ΔH_a) refer to?
-The standard enthalpy of atomization (ΔH_a) refers to the heat absorbed when one mole of a compound is converted into its gaseous atoms.
What happens during the standard enthalpy of fusion (ΔH_fus)?
-During the standard enthalpy of fusion (ΔH_fus), heat is absorbed when one mole of a solid substance melts into a liquid.
How is the standard enthalpy of vaporization (ΔH_vap) defined?
-The standard enthalpy of vaporization (ΔH_vap) is defined as the heat absorbed when one mole of a liquid vaporizes into a gas.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes
5.0 / 5 (0 votes)