Hukum Termodinamika, Bagian 3: Termokimia
Summary
TLDRIn this video, Professor Habiburrahman from the Department of Chemical Engineering at the University of Indonesia discusses thermochemistry, focusing on enthalpy, standard enthalpy changes, and the laws related to thermodynamic processes. The content includes topics such as the definition of standard states, various enthalpy changes (vaporization, fusion, and formation), Hess's Law, and Kirchhoff's Law. The lecture also emphasizes the importance of temperature and pressure in chemical reactions and provides examples to help students understand the practical applications of these principles in thermochemistry.
Takeaways
- 😀 The video covers thermochemistry, particularly focusing on concepts such as enthalpy changes, standard enthalpy of reactions, and Hess's Law.
- 😀 The standard state of a chemical species is defined as its pure form at a specified temperature (usually 298 K) and at a pressure of 1 bar.
- 😀 Different types of enthalpy changes, such as vaporization, fusion, and formation, are discussed along with their symbolic representations and their importance in communication.
- 😀 Specific examples of enthalpy changes for water, such as from liquid to gas and from solid to liquid, are provided to illustrate how enthalpy changes depend on temperature and phase.
- 😀 The concept of enthalpy changes in standard conditions is illustrated using reactions at different temperatures (e.g., 0°C and 100°C for water's phase changes).
- 😀 Hess's Law is introduced, explaining that the total enthalpy change of a reaction is the sum of enthalpy changes of individual steps.
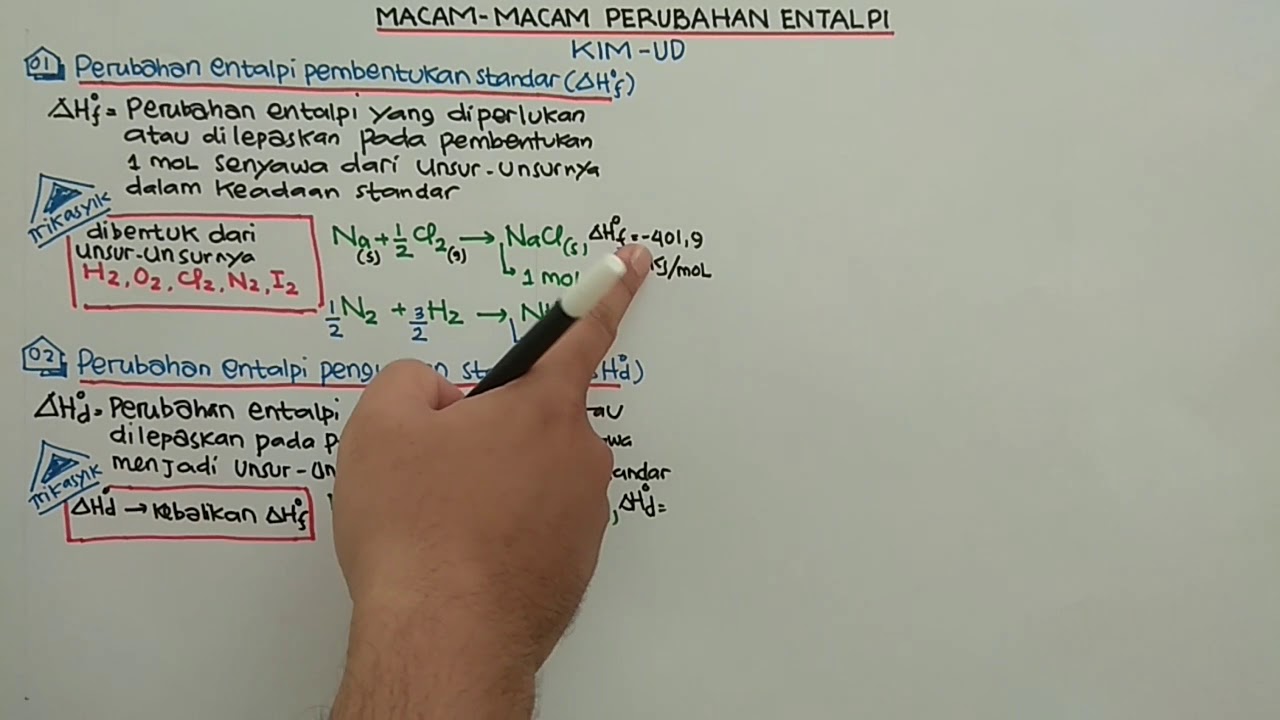
- 😀 Standard enthalpy of formation is explained, emphasizing that the formation enthalpy of elements in their most stable form at standard conditions is zero.
- 😀 The relationship between enthalpy changes and the stoichiometry of a reaction is discussed, including how to calculate the enthalpy change using standard formation enthalpies.
- 😀 The video explains how enthalpy can be linked with temperature changes through a formula that includes the molar heat capacity and the temperature difference.
- 😀 The importance of specifying the temperature and pressure when discussing enthalpy changes is emphasized to avoid ambiguity and ensure accurate reporting.
Q & A
What is the definition of standard state in thermochemistry?
-The standard state of a chemical species at a specific temperature is its pure form at a pressure of 1 bar. The temperature must also be specified for clarity when discussing thermodynamic properties.
What is the relationship between enthalpy change and temperature?
-Enthalpy change can depend on temperature. For instance, enthalpy changes like vaporization or fusion are often reported at specific temperatures, such as 100°C (373K) for water's vaporization or 0°C (273K) for melting.
How are enthalpy changes for different processes standardized?
-Enthalpy changes are standardized by considering specific conditions like temperature and pressure (1 bar). For example, water's transition from liquid to gas at 100°C involves the enthalpy of vaporization at 373K and 1 bar pressure.
What is Hess's Law and how is it applied?
-Hess's Law states that the total enthalpy change of a reaction is the sum of the enthalpy changes of the individual steps of the reaction. This law allows us to calculate the enthalpy change of a complex reaction by combining the enthalpy changes of simpler reactions.
What is the significance of the standard enthalpy of formation?
-The standard enthalpy of formation refers to the enthalpy change when one mole of a compound is formed from its elements in their standard states under specified conditions, typically at 298K and 1 bar pressure.
How does temperature affect the enthalpy of a substance?
-The enthalpy of a substance can change with temperature. The relationship can be expressed using the formula for enthalpy at a different temperature, which incorporates the heat capacity and temperature difference between two states.
What is the role of heat capacity in enthalpy changes?
-Heat capacity plays a crucial role in calculating enthalpy changes, as it affects how much heat is absorbed or released during a process. The change in enthalpy at different temperatures can be determined using the specific heat capacities of the substances involved.
What does the enthalpy of formation tell us about a compound?
-The enthalpy of formation indicates the energy required to form a compound from its constituent elements in their standard states. This value is crucial for determining the overall energy changes in reactions involving that compound.
What is the significance of positive and negative values in enthalpy changes?
-A positive enthalpy change indicates an endothermic process (energy absorbed), while a negative value indicates an exothermic process (energy released). These values are important for understanding the energy dynamics of chemical reactions.
How does the standard state of a substance influence its enthalpy calculation?
-The standard state of a substance, which is its most stable form at 1 bar pressure and a specified temperature, directly affects the enthalpy calculations. For example, oxygen in its standard state is a gas, and the enthalpy for reactions involving oxygen is based on this form.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)