Korosi | Kimia SMA
Summary
TLDRIn this video, we explore the topic of corrosion, particularly focusing on rust formation in iron. Corrosion is a redox reaction where metals interact with environmental substances, producing unwanted compounds. We delve into the electrochemical process of iron rusting, where iron oxidizes at the anode and oxygen reduces at the cathode. The video also covers factors accelerating corrosion, such as humidity, temperature, and electrolytes. Methods to prevent corrosion, like cathodic protection and protective coatings, are discussed, providing practical solutions to prolong the life of metal structures. Thank you for watching. Wassalamualaikum warahmatullahi wabarakatuh.
Takeaways
- 🔬 Corrosion (also known as rusting) is a redox reaction between a metal and substances in its environment, producing unwanted compounds.
- 🧪 Corrosion is an electrochemical process where the metal undergoes oxidation, typically forming metal oxides or carbonates, while oxygen in the air undergoes reduction.
- ⚙️ The most common example of corrosion is rust formation on iron, resulting in a reddish-brown solid with the chemical formula Fe2O3·xH2O.
- 🔗 Silver tarnishing and the greenish patina on copper are also examples of corrosion.
- 💧 For rusting to occur, there must be direct contact between iron, water, and oxygen. Iron oxidizes at the anode, while oxygen is reduced at the cathode.
- ⚡ The chemical reaction at the anode involves Fe becoming Fe2+ by releasing electrons, and at the cathode, oxygen reacts with water and electrons to form OH-. These reactions drive the corrosion process.
- 🔥 Several factors accelerate corrosion: high humidity (increases water and oxygen concentration), higher temperature (speeds up reaction rates), and the presence of electrolytes (e.g., salt).
- 🥶 Acidic environments (pH below 7) can also speed up corrosion, and galvanic coupling with less reactive metals can further enhance the process.
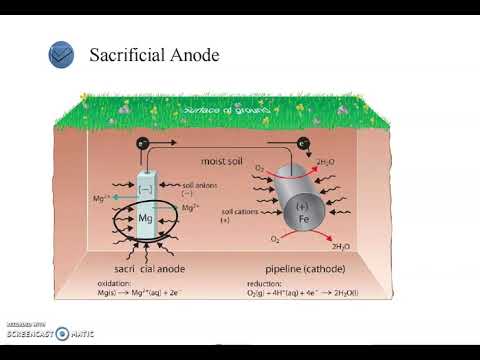
- 🛡️ Corrosion control methods include cathodic protection (using more reactive metals like magnesium) and applying protective coatings (e.g., paint, oil, plastic, zinc, tin, or chromium).
- 🔧 Zinc coating (galvanization) protects iron by making zinc oxidize instead of iron, while tin and chromium coatings prevent contact with water and oxygen, though damaged tin can accelerate rusting.
Q & A
What is corrosion, as described in the video?
-Corrosion, also known as rusting, is a redox reaction between a metal and substances in its environment, resulting in undesirable compounds. It is an electrochemical process where metal undergoes oxidation, usually forming metal oxides or carbonates.
What is the redox reaction involved in the formation of rust on iron?
-In the formation of rust on iron, iron (Fe) is oxidized at the anode, forming Fe2+ ions and releasing electrons. Oxygen (O2) in the presence of water is reduced at the cathode, forming hydroxide ions (OH-). The overall reaction results in the formation of iron oxides (Fe2O3·xH2O), which is rust.
What are some common examples of corrosion mentioned in the video?
-Examples of corrosion include the formation of rust on iron, tarnishing of silver, and the greenish patina that forms on copper surfaces.
What factors accelerate the process of corrosion?
-Factors that accelerate corrosion include high humidity (increased water and oxygen concentration), higher temperatures, the presence of electrolytes such as salt, acidic pH (lower than 7), and Galvanic coupling where iron is in contact with a less reactive metal.
How does temperature influence the rate of corrosion?
-Higher temperatures increase the rate of chemical reactions, including corrosion. Therefore, elevated temperatures speed up the corrosion process.
What is Galvanic coupling, and how does it affect corrosion?
-Galvanic coupling occurs when iron is in contact with a less reactive metal or a metal with a higher reduction potential than iron. In this situation, iron corrodes faster because the less reactive metal tends to protect itself, forcing iron to oxidize more readily.
What is cathodic protection, and how does it prevent corrosion?
-Cathodic protection involves connecting iron to a more reactive metal, like magnesium. The more reactive metal oxidizes (sacrifices itself) while iron is reduced, preventing it from corroding. This method protects iron from oxidation.
What are some ways to prevent or reduce corrosion?
-Corrosion can be prevented or reduced through methods such as cathodic protection, applying protective coatings like paint, oil, plastic, or metal plating (using tin, zinc, or chromium), and reducing exposure to water and oxygen.
Why is zinc plating (galvanization) effective in protecting iron from corrosion?
-Zinc plating, or galvanization, is effective because zinc has a lower reduction potential than iron, meaning zinc will oxidize before iron. This sacrificial protection prevents iron from rusting.
What happens if a tin coating on iron is damaged?
-If a tin coating on iron is damaged, tin, which is more easily reduced than iron, can accelerate the oxidation of exposed iron, leading to faster rust formation.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenant5.0 / 5 (0 votes)