Why Does Metal Rust? - Reactions Q&A
Summary
TLDRIn this video, Sophia explains the science behind why metal objects corrode, particularly focusing on rust formation. Corrosion is the result of a redox reaction, where oxidation and reduction occur simultaneously. Iron, a metal prone to rusting, undergoes a reaction with water as an electrolyte, resulting in the formation of rust, or ferric oxide. The process occurs because rusting is the thermodynamically preferred state for iron, even though humans use it in its pure metallic form. Sophia wraps up by inviting viewers to drop more questions for future explanations.
Takeaways
- 😀 Corrosion is caused by a redox reaction, which involves two simultaneous reactions: reduction and oxidation.
- 😀 In a reduction reaction, atoms or molecules gain electrons, while in oxidation, they lose electrons.
- 😀 The term 'redox' is a combination of reduction and oxidation reactions.
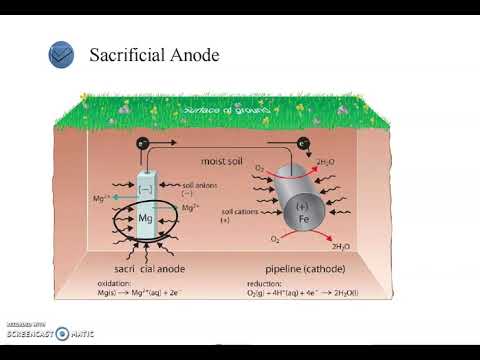
- 😀 A common example of corrosion is rusting, which occurs when iron reacts with water and oxygen.
- 😀 Rust formation requires three ingredients: an anode (iron), a cathode (oxygen), and an electrolyte solution (water).
- 😀 Water acts as the electrolyte solution in the corrosion process, facilitating the movement of ions between the cathode and anode.
- 😀 Iron rusts to form hydrated ferric oxide (Fe2O3⋅nH2O), a product of the redox reaction.
- 😀 Iron and other metals that rust are thermodynamically inclined to form rust, as it's their preferred state.
- 😀 Humans force metals to remain in their pure form for practical use, but rusting is the metal’s natural state.
- 😀 Rusting serves as the metal’s way of showing its preference for a stable, thermodynamically favored state.
- 😀 The script invites viewers to submit more questions for future videos on related topics.
Q & A
What is corrosion and what causes it?
-Corrosion is a process caused by a redox reaction, which involves both reduction and oxidation reactions happening simultaneously. It typically leads to the degradation of metals, like rusting in iron.
What is a reduction reaction?
-A reduction reaction occurs when atoms or molecules gain electrons. This is one part of the redox process.
What happens during oxidation?
-Oxidation is the process where atoms or molecules lose electrons. It is the second part of the redox reaction, which works alongside reduction.
What does the term 'redox' mean?
-The term 'redox' is a combination of reduction and oxidation reactions that occur simultaneously in processes like corrosion and battery operation.
How does rust form on metal objects?
-Rust forms when iron or other metals react with water (which acts as an electrolyte) and oxygen, causing a redox reaction. This results in the formation of hydrated ferric oxide (Fe2O3⋅nH2O), commonly known as rust.
What are the three key components required for rust to form?
-The three key components required for rusting are: 1) An anode (iron), which gives up electrons, 2) A cathode (oxygen), which accepts electrons, and 3) An electrolyte solution (usually water) that shuttles ions between the anode and cathode.
Why do some metal objects rust after being left out in the rain?
-Rainwater acts as the electrolyte solution that facilitates the redox reactions between iron and oxygen, leading to the formation of rust on the metal surface.
What is ferric oxide and how does it relate to rust?
-Ferric oxide (Fe2O3) is the chemical compound that forms when iron undergoes corrosion. When it combines with water, it forms hydrated ferric oxide (Fe2O3⋅nH2O), which is the reddish-brown substance known as rust.
Why does iron prefer to form rust rather than stay in its pure form?
-Rusting is the thermodynamically favored state for iron. In other words, iron naturally prefers to oxidize into rust over time because it is a more stable form than its pure metallic state.
Are all metals prone to rusting?
-Not all metals rust in the same way. Rusting specifically refers to the corrosion of iron and steel. Other metals may corrode differently, but the process of corrosion can affect many different materials.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)