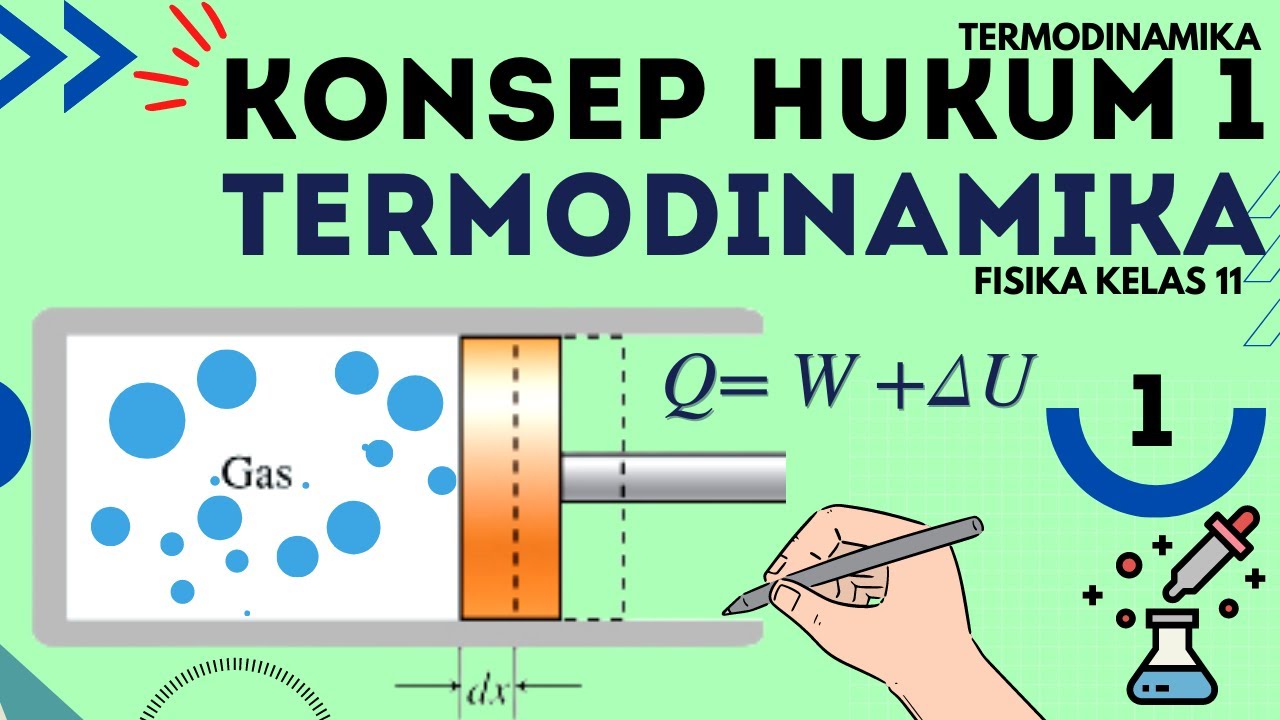
12th Science | Thermodynamics in 1 Shot | HSC | MHT-CET
Summary
TLDRThe video introduces the topic of thermodynamics in a conversational style, explaining key concepts like heat transfer, internal energy, temperature, and thermodynamic processes. It covers fundamental laws like the first and zeroth laws of thermodynamics, thermal equilibrium, and the relationship between heat, temperature, and work. The speaker simplifies these concepts using relatable examples such as the cooling of a cup of tea. The discussion also touches on thermodynamic systems, processes like isothermal and adiabatic changes, and the significance of equilibrium. The video encourages students to focus on understanding these principles for exams.
Takeaways
- 🌡️ Thermodynamics is the study of heat and its relationship to other forms of energy.
- 🔥 Heat transfer occurs between substances at different temperatures, moving from higher to lower temperature objects.
- 💡 The first law of thermodynamics is essentially the law of conservation of energy: energy cannot be created or destroyed.
- 🛑 A system is considered in thermal equilibrium when its temperature is uniform across all parts and no heat flows in or out.
- 🔄 A reversible process can be undone without any net change to the system or surroundings, while irreversible processes cannot.
- 🔧 Work done by a system is dependent on the initial, final, and intermediate states of the system.
- 📏 Pressure, volume, and temperature are the key variables that define the state of a thermodynamic system.
- ⚖️ The PV diagram graphically represents the relationship between pressure and volume in a thermodynamic process.
- 🚫 In an adiabatic process, no heat is transferred to or from the system, meaning changes in internal energy are entirely due to work done.
- 📚 The ideal gas law (PV = nRT) describes the behavior of ideal gases, particularly how pressure, volume, and temperature are interrelated.
Q & A
What is thermodynamics?
-Thermodynamics is the branch of physics that deals with concepts of heat, temperature, and energy. It studies how heat is transferred and how it interacts with other forms of energy.
What happens to heat when it is transferred from a hot object to a cold one?
-Heat naturally flows from a substance at a higher temperature to one at a lower temperature until thermal equilibrium is reached, meaning both objects are at the same temperature.
What is thermal equilibrium?
-Thermal equilibrium occurs when two substances at different temperatures reach the same temperature, resulting in no further heat transfer between them.
What are state variables in thermodynamics?
-State variables are properties like pressure, volume, and temperature, which describe the state of a thermodynamic system.
What is the first law of thermodynamics?
-The first law of thermodynamics, also known as the law of energy conservation, states that the total energy of a system is constant. Energy can be transferred between systems but cannot be created or destroyed.
What is a thermodynamic system?
-A thermodynamic system is a group of objects or a collection that can exchange energy with its surroundings. It is defined by variables like temperature, pressure, and volume.
What are reversible and irreversible processes?
-A reversible process is a thermodynamic process that can be reversed without leaving any net change in either the system or its surroundings. An irreversible process, on the other hand, cannot be reversed without increasing entropy or causing energy loss.
What is internal energy?
-Internal energy is the total energy contained within a system, which includes both the kinetic energy of the system's molecules and potential energy from interactions between molecules.
What is the difference between work and heat in thermodynamics?
-In thermodynamics, work is energy transferred to or from a system by mechanical means (such as moving a piston), while heat is energy transferred due to a temperature difference between the system and its surroundings.
What is the equation of state for an ideal gas?
-The equation of state for an ideal gas is given by PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes
5.0 / 5 (0 votes)