1ª LEI DA TERMODINÂMICA | Resumo de Física para o Enem
Summary
TLDRIn this educational video, Professor Rossetto introduces key concepts of thermodynamics, focusing on heat and temperature. He explains that heat is the transfer of energy between objects of different temperatures, while temperature measures the kinetic energy of particles. The first law of thermodynamics is covered, highlighting energy conservation and the relationship between heat, work, and internal energy. The video discusses different thermodynamic processes, including isothermal, isovolumetric, and adiabatic transformations, with relatable examples like pressure cookers and temperature changes when touching different objects. The goal is to help students understand these concepts in a practical, engaging way.
Takeaways
- 😀 Heat is the transfer of thermal energy from a hotter object to a cooler one, while temperature measures the movement of particles within an object.
- 😀 We don't 'have' heat; we sense it when energy is lost (cold) or gained (warm).
- 😀 Touching a cold doorknob feels cold because it draws energy away from your hand.
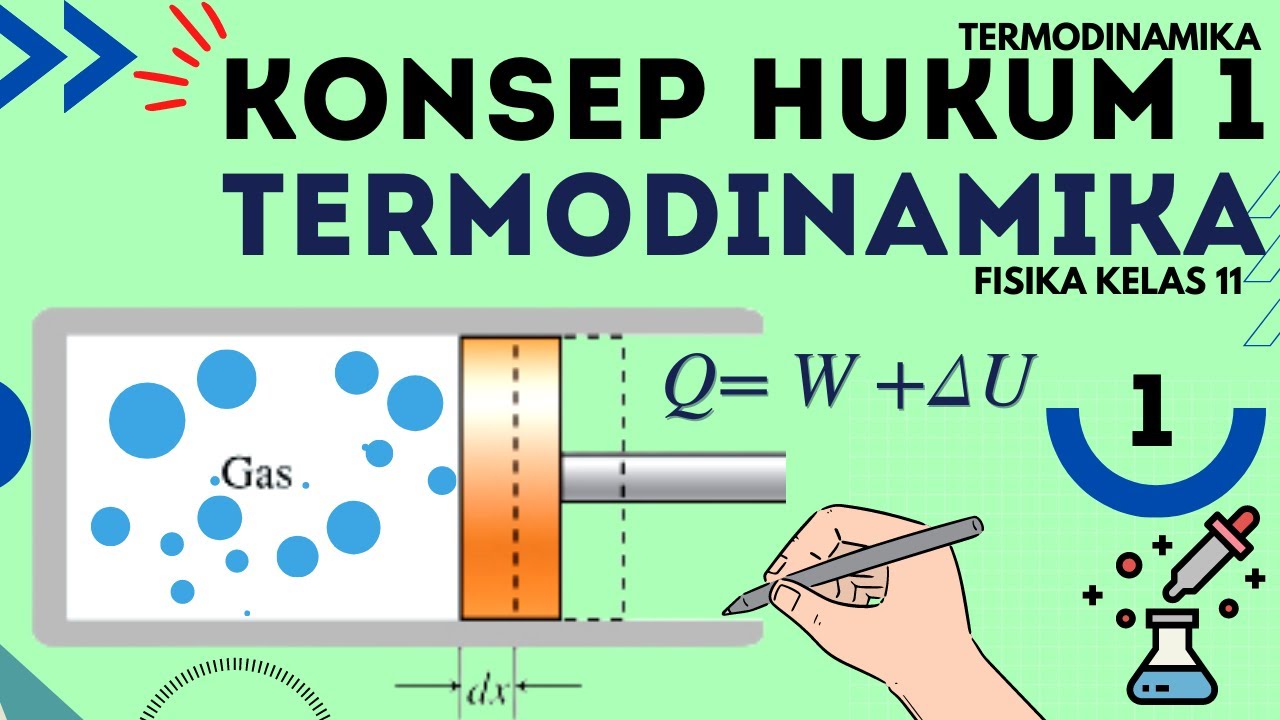
- 😀 The First Law of Thermodynamics states that heat added to a system either increases its internal energy or performs work (expansion/compression).
- 😀 In an isothermal process, temperature remains constant, and all heat added is converted into work, though this is theoretical in real life.
- 😀 Isovolumetric processes occur at constant volume, so no work is done, and all heat increases internal energy (temperature changes).
- 😀 In an adiabatic process, there is no heat exchange with the surroundings. A rapid gas expansion cools it, while rapid compression heats it up.
- 😀 An example of an isothermal process is slowly squeezing a balloon, where volume decreases, pressure increases, but temperature stays the same.
- 😀 Isovolumetric examples include a pressure cooker or a car tire, where pressure increases as the gas inside heats up, but volume stays constant.
- 😀 Thermal sensations can differ between materials (e.g., wood vs. marble) even at the same temperature due to differences in heat conduction.
- 😀 Understanding thermodynamics helps explain energy transformations in everyday life, from balloons to car tires.
Q & A
What is the difference between heat and temperature?
-Heat is the energy transferred between bodies of different temperatures, while temperature measures the kinetic energy of particles within a substance. Heat flows from a higher to a lower temperature body, while temperature indicates how much the particles are agitated.
How does the sensation of heat occur?
-The sensation of heat occurs when we gain energy, and the sensation of cold happens when we lose energy. For example, touching a hot object transfers energy to your skin, making you feel hot, while touching a cold object causes energy to transfer away, making you feel cold.
What is the first law of thermodynamics?
-The first law of thermodynamics is the law of conservation of energy. It states that the total energy in an isolated system is constant. In thermodynamic processes, heat supplied to a system can either be converted into internal energy or work.
What happens in an isothermal process?
-In an isothermal process, the temperature of the system remains constant. All the heat supplied to the system is converted into work, and there is no change in internal energy.
What defines an isovolumetric process?
-An isovolumetric process occurs when the volume of the system remains constant. Since there is no volume change (ΔV = 0), no work is done, and all the heat supplied is converted into a change in internal energy, which results in heating or cooling.
What is an adiabatic process?
-An adiabatic process is one in which no heat is exchanged with the surroundings. In such processes, work done on or by the system results in a temperature change. For example, quickly compressing or expanding a gas can either heat or cool it.
Can you explain how a deodorant spray illustrates an adiabatic process?
-When you spray deodorant, the gas inside expands rapidly, causing a decrease in temperature. This happens because no heat is exchanged with the environment, and the rapid expansion of the gas leads to cooling (adiabatic expansion).
Why does compressing a gas quickly result in heating?
-When you compress a gas rapidly, the work done on the gas increases its internal energy, causing the temperature to rise. This is because compressing the gas causes the particles to collide more frequently, transferring kinetic energy and generating heat.
How does a balloon provide an example of an isothermal process?
-If a balloon is squeezed slowly, the volume decreases, and the pressure increases. If the temperature remains constant during this process, it demonstrates an isothermal transformation, where all heat supplied is converted into work without changing the internal energy of the system.
What happens inside a pressure cooker or car tire that relates to thermodynamic processes?
-In a pressure cooker or car tire, the volume remains constant while the temperature of the gas inside increases. This results in an increase in pressure, as the gas molecules move faster when heated, demonstrating an isovolumetric process where the heat supplied leads to a rise in pressure without changing the volume.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Calorimetria - Aula 01 (Calor sensível)

Termologia - Conceitos Iniciais - Aula 01

Termodinamika - Fisika Kelas 11 (Kurikulum 2013 Revisi) - Quipper Video
Hukum 1 Termodinamika : Termodinamika Fisika Kelas 11 - Part 1

Calor sensível e calor latente conceitos para entender definitivamente

TROCAS DE CALOR COM MUDANÇA DE FASE - TERMOLOGIA - Aula 8 - Prof. Boaro
5.0 / 5 (0 votes)