Superacids and Superbases
Summary
TLDRThe video script delves into the concept of acidity, explaining that in aqueous solutions, the hydronium ion represents the strongest acid. It discusses the transition to non-aqueous solutions and the use of the Hammett function (H0) to measure acidity beyond the pH scale. The script introduces super acids like fluorosulfonic acid and mixtures involving HF and SbF5, which can even protonate hydrocarbons. It also touches on super bases, such as lithium amide, and their reactivity with water, emphasizing that these substances operate in anhydrous conditions.
Takeaways
- 🌡️ The strongest acid in water is the hydronium ion, and no stronger acid can override its strength in an aqueous solution.
- 🔄 To achieve higher acidity, one must move from equal solutions to other solvents, such as pure acid solutions, which can be referred to as super acids.
- 📊 For non-aqueous solutions, the Hammett function (H0) is used instead of pH to measure the strength of acids and bases beyond the usual pH scale of 0 to 14.
- 🚫 Pure sulfuric acid has a relative strength, not a pH of minus 12, indicating an extremely strong acid that cannot be represented by a simple hydrogen ion concentration.
- 🔝 Adding more SO3 to sulfuric acid can create H2S2O7, which has a Hammett function of minus 15, showing an increase in acidity.
- 🧪 Replacing an oxygen in sulfuric acid with fluorine results in fluorosulfonic acid, which has a Hammett function of minus 15.1, indicating a slight increase in acidity.
- 🌐 Even weak acids like hydrogen fluoride can become super acids when in their pure liquid state, forming the H2F cation.
- 🔬 The addition of antimony pentafluoride (SbF5) to solutions can further increase their acidity, such as in the case of chlorosulfonic acid plus SbF5, which has a Hammett function of minus 19.
- ✨ The strongest known acid is a mixture of HF and SbF5, which can protonate hydrocarbons, including methane, which normally has no acidic properties.
- 🔄 In the absence of water, super bases like lithium amide can be stronger than hydroxide, but they react instantly with water to form hydroxide ions.
- 🧪 Nitric acid in sulfuric acid forms NO2+ and H3O+ cations, which are important in organic chemistry for protonation reactions.
Q & A
What is the strongest acid in water?
-The strongest acid in water is the hydronium ion (H3O+). No matter what stronger acid is added, it will instantly become hydronium ion and the corresponding anion in the aqueous solution.
Can the strength of hydronium ion be overridden by other acids?
-No, the strength of the hydronium ion cannot be overridden by other acids in an aqueous solution.
How can we achieve higher acidity than that of hydronium ion?
-To achieve higher acidity, one must use pure acid solutions instead of aqueous solutions and consider super acids.
What are super acids and how are they different from regular acids?
-Super acids are acids that are stronger than 100% sulfuric acid. They are capable of protonating hydrocarbons and are measured using the Hammett function (H0) instead of pH.
What is the Hammett function (H0) and how is it used?
-The Hammett function (H0) is a scale used to measure the acidity of non-aqueous solutions, extending beyond the usual pH range of 0 to 14.
What is the pH of pure sulfuric acid?
-The pH of pure sulfuric acid is not accurately represented by the pH scale; instead, it is described as having a relative strength of minus 12, indicating its extreme acidity.
How does the addition of SO3 affect the acidity of sulfuric acid?
-Adding more SO3 to sulfuric acid can form H2S2O7, which has a Hammett function of minus 15, indicating an even stronger acidity.
What is fluorosulfonic acid and how strong is it?
-Fluorosulfonic acid is a super acid with a Hammett function of minus 15.1, indicating it is one of the strongest acids known.
Why can weak acids like hydrogen fluoride become stronger in the absence of water?
-In the absence of water, weak acids like hydrogen fluoride can form cations such as H2F+, which are much stronger than the hydronium ion, making the acid significantly stronger.
What is 'magic acid' and why is it called so?
-Magic acid is a mixture of chlorosulfonic acid and antimony pentafluoride (SbF5) with a Hammett function of minus 19. It is called 'magic' because it can protonate hydrocarbons, including a candle, which dissolves when accidentally placed in the solution.
What is the strongest acid known today?
-The strongest acid known today is a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), which can protonate even methane, a molecule with no available lone pair electrons for protonation.
How do super bases differ from regular bases in water?
-Super bases, such as lithium amide, are much stronger than regular bases like hydroxide ions in water. They are typically used in the absence of water to avoid immediate reaction with it to form hydroxide ions.
What happens when super acids and super bases react with water?
-Super acids and super bases react instantaneously with water to form hydroxide ions (OH-) and hydronium ions (H3O+), respectively.
Why is the geometry of the protonated methane molecule complex?
-The geometry of protonated methane is complex because one of the hydrogen atoms is connected to two other hydrogen atoms, leading to a structure that could be described as having symmetry between D3h or C4v.
What is the significance of the reaction between nitric acid and sulfuric acid in organic chemistry?
-The reaction between nitric acid and sulfuric acid is significant because it forms NO2+ and H3O+ cations, which are important in organic chemistry for their ability to act as strong proton donors.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

Arrhenius definition of acids and bases | Biology | Khan Academy

Brønsted–Lowry acids and bases | Chemical reactions | AP Chemistry | Khan Academy

The pH scale | Acids and bases | AP Chemistry | Khan Academy
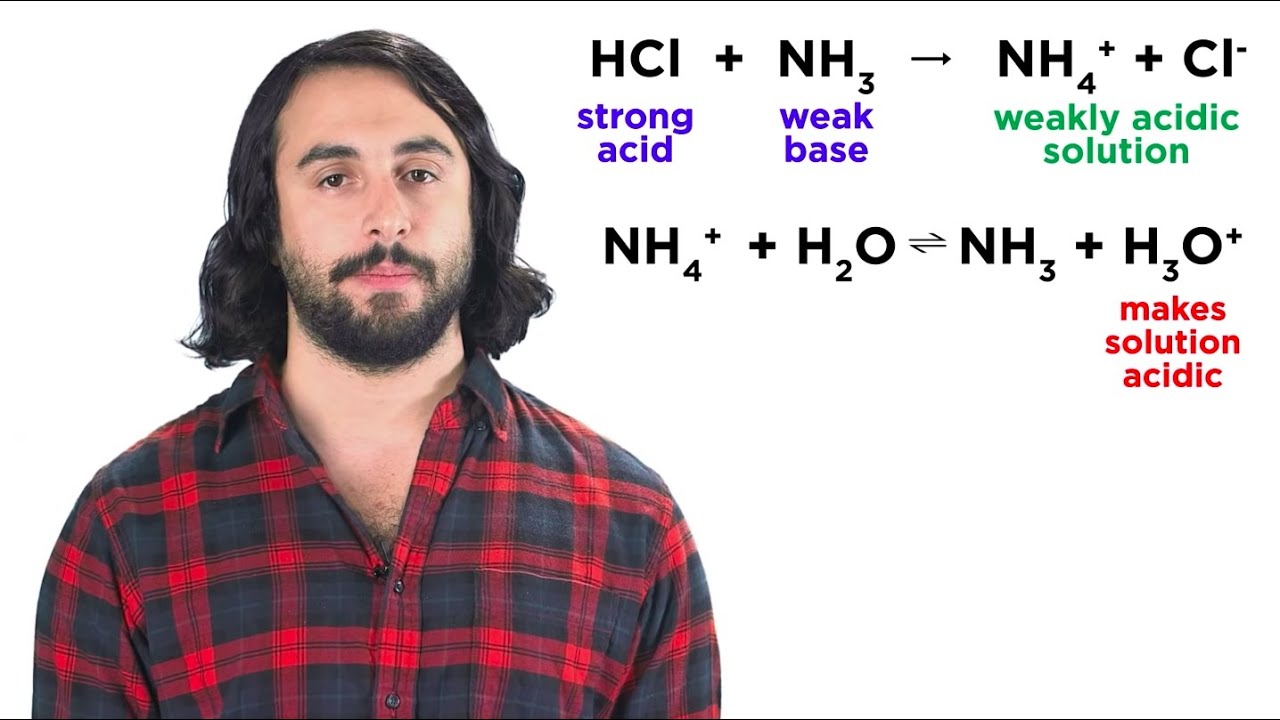
Neutralization Reactions

The Chemistry of Ocean Acidification and its Consequences for Ocean Life
pH and pOH: Crash Course Chemistry #30
5.0 / 5 (0 votes)
