pH and pOH: Crash Course Chemistry #30
Summary
TLDRThis Crash Course Chemistry episode delves into the concept of pH, explaining its significance in measuring the acidity or alkalinity of a solution. The video clarifies the origin of the term 'pH' and its mathematical definition as the negative logarithm of hydrogen ion concentration. It explores the unique properties of water, which can act as both an acid and a base, and introduces the water dissociation constant (Kw). The episode also covers the pH scale, differentiating between strong and weak acids and bases, and introduces pOH. It concludes with a fascinating fact: the sum of pH and pOH in any aqueous solution always equals 14.
Takeaways
- 🧼 pH balance is a concept related to personal grooming products and is essential for maintaining the skin's health.
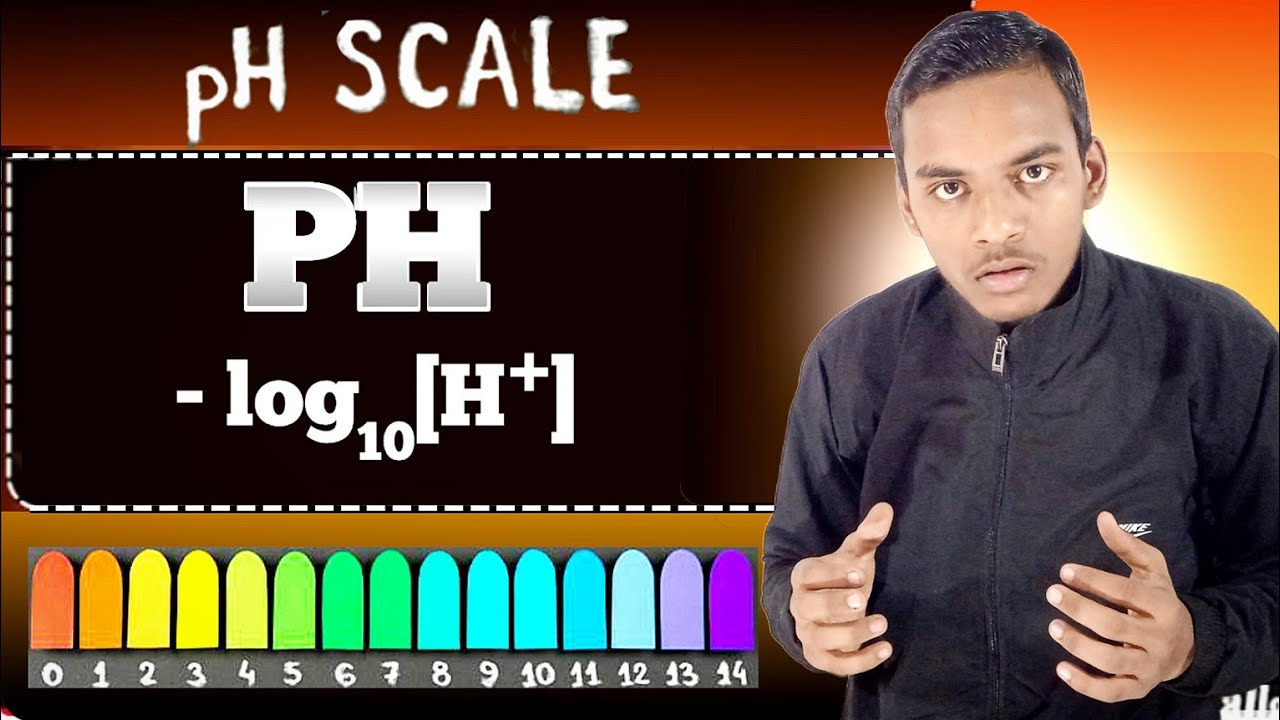
- 🔍 pH stands for 'power of hydrogen', and it measures the concentration of hydrogen ions in a solution, indicating whether it is acidic or basic.
- 🤔 The 'p' in pH is of uncertain origin, possibly derived from 'power' in various languages, while 'H' stands for hydrogen.
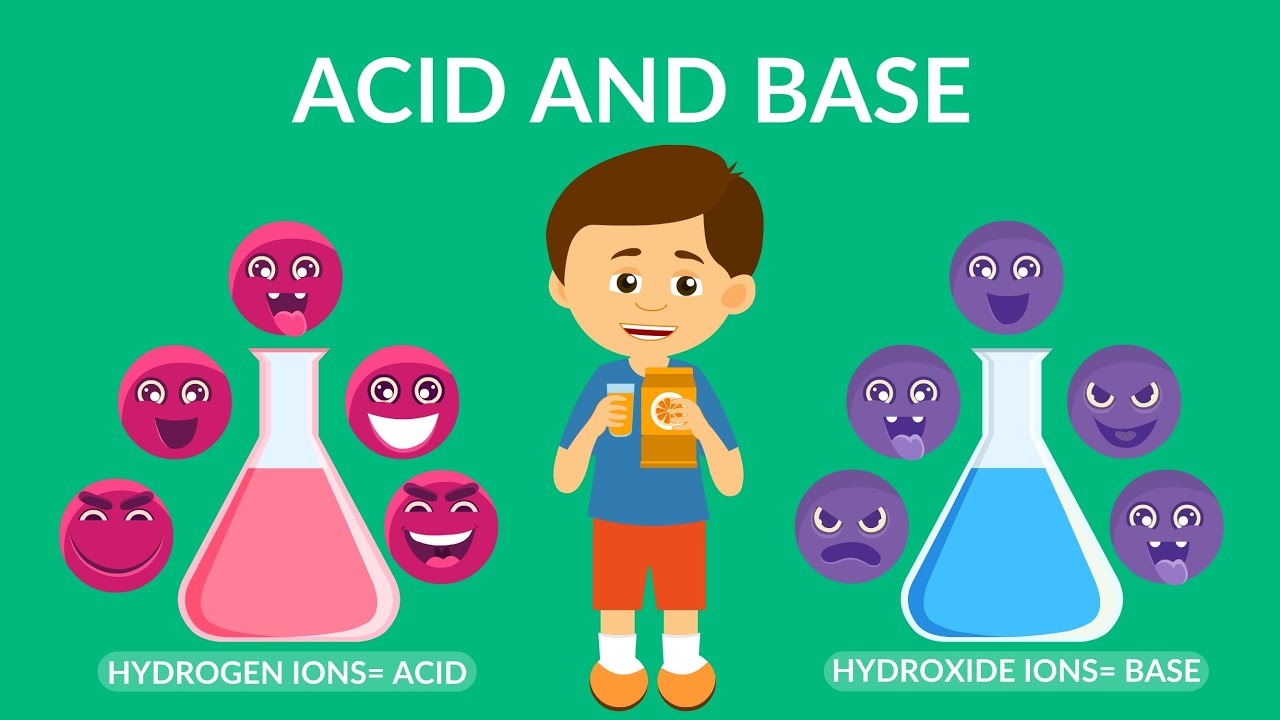
- 💧 Water is unique as it can act as both an acid and a base due to its ability to ionize into hydronium (H3O+) and hydroxide (OH-) ions.
- ⚖️ The pH scale ranges from 0 to 14, with 7 being neutral, below 7 being acidic, and above 7 being basic.
- 📉 pH is the negative logarithm (base 10) of the hydrogen ion concentration, which helps in dealing with very small numbers in chemistry.
- 🔋 The water dissociation constant (Kw) is a special equilibrium constant that equals 1.0 x 10^-14 at 25 degrees Celsius.
- 🔄 The equilibrium concentrations of hydronium and hydroxide ions in pure water are equal and can be calculated using the square root of Kw.
- 📊 pH and pOH are inversely related, and their sum always equals 14, reflecting the product of hydrogen and hydroxide ion concentrations in any aqueous solution.
- 🧪 Strong acids and bases have a significant effect on pH, while weak acids and bases have a lesser effect due to their partial ionization.
Q & A
What does the term 'pH' stand for and why is it written with a lowercase 'p' and a capital 'H'?
-The term 'pH' stands for 'power of hydrogen,' referring to the concentration of hydrogen ions in a solution. The 'p' is lowercase because its meaning is not definitively known; it was coined by the Danish chemist Søren Sørensen, who did not explain its origin. Some speculate it might come from a word like 'puissance' or 'pondus,' but it's more likely a convention to differentiate test solutions. The 'H' stands for hydrogen, as hydrogen ions are central to the behavior of acids and bases.
Why is water considered special in the context of pH?
-Water is considered special in the context of pH because it can act as both an acid and a base. This is due to its ability to dissociate into hydronium (H3O+) and hydroxide (OH-) ions, which allows it to release or consume hydrogen ions (protons), respectively.
What is the mathematical definition of pH?
-The pH of a substance is defined mathematically as the negative of the base 10 logarithm of the concentration of hydrogen ions in the solution. This means that pH is a measure of the relative acidity or basicity of a solution based on the concentration of hydrogen ions present.
What is the significance of the water dissociation constant (Kw) and what is its value?
-The water dissociation constant (Kw) is significant because it represents the equilibrium constant for the dissociation of water into hydronium and hydroxide ions. Its value is 1.0 x 10^-14, indicating the product of the concentrations of these ions in pure water at equilibrium.
How does the pH scale relate to the acidity or basicity of a solution?
-The pH scale is a measure of the acidity or basicity of a solution, with a pH of 7 being neutral. Values below 7 indicate acidity, with lower numbers being more acidic, while values above 7 indicate basicity, with higher numbers being more basic.
What is the relationship between pH and pOH?
-The relationship between pH and pOH is that they are complementary measures in aqueous solutions. The sum of the pH and pOH of a solution is always 14, which is derived from the water dissociation constant (Kw) and the fact that the product of the concentrations of hydrogen ions and hydroxide ions equals Kw.
Why are logarithms used in the calculation of pH?
-Logarithms are used in the calculation of pH because they provide a convenient way to express and compare very large or very small numbers, which are common in chemistry when dealing with the concentrations of ions in solutions.
What is the difference between strong and weak acids or bases in terms of their pH?
-Strong acids or bases completely or almost completely dissociate in water, resulting in a high concentration of hydrogen ions (for acids) or a low concentration of hydrogen ions (for bases), leading to low or high pH values, respectively. Weak acids or bases only partially dissociate, resulting in lower concentrations of ions and therefore higher or lower pH values compared to strong acids or bases.
How does the addition of an acid or a base affect the pH of water?
-Adding an acid to water increases the concentration of hydrogen ions, thus lowering the pH. Conversely, adding a base to water decreases the concentration of hydrogen ions by consuming them, which raises the pH.
What is the practical range of pH values for most substances encountered in everyday life?
-The practical range of pH values for most substances encountered in everyday life is typically between 0 and 14, with neutral pH being around 7. However, most substances fall within a narrower range, often between 4 and 9, due to the natural buffering capacity of many systems and the stability of biological and environmental conditions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Acidity: Crash Course Organic Chemistry #11

Soil Chemistry Introduction
What is PH?🤔 PH क्या होता है ll How find it ll Chemistry ll importance in our daily life ll

Pengantar Oseanografi - Acidity, Alkalinity dan Dissolve Gases
Acid and Base | Acids, Bases & pH | Video for Kids

What Is The pH Scale | Acids, Bases & Alkalis | Chemistry | FuseSchool
5.0 / 5 (0 votes)