An Electron's Quantum Address: Quantum Numbers
Summary
TLDRThis video unpacks the elegant system of quantum numbers, which act as a precise 'address' for electrons inside atoms. Using the analogy of a city, street, house, and resident, it explains the four quantum numbers: principal (n) for energy level, angular momentum (L) for orbital shape, magnetic (M_L) for orbital orientation, and spin (M_S) for electron spin. The video also highlights the Pauli exclusion principle, which ensures no two electrons share the same quantum address, limiting orbitals to two electrons. Ultimately, these principles govern electron configurations, chemical bonding, and the structure of the periodic table, revealing the profound rules shaping matter itself.
Takeaways
- 😀 Quantum numbers provide a unique 'address' for electrons, describing their exact position and behavior within an atom.
- 😀 Atoms are mostly empty space, yet quantum numbers help pinpoint where an electron is likely to be found.
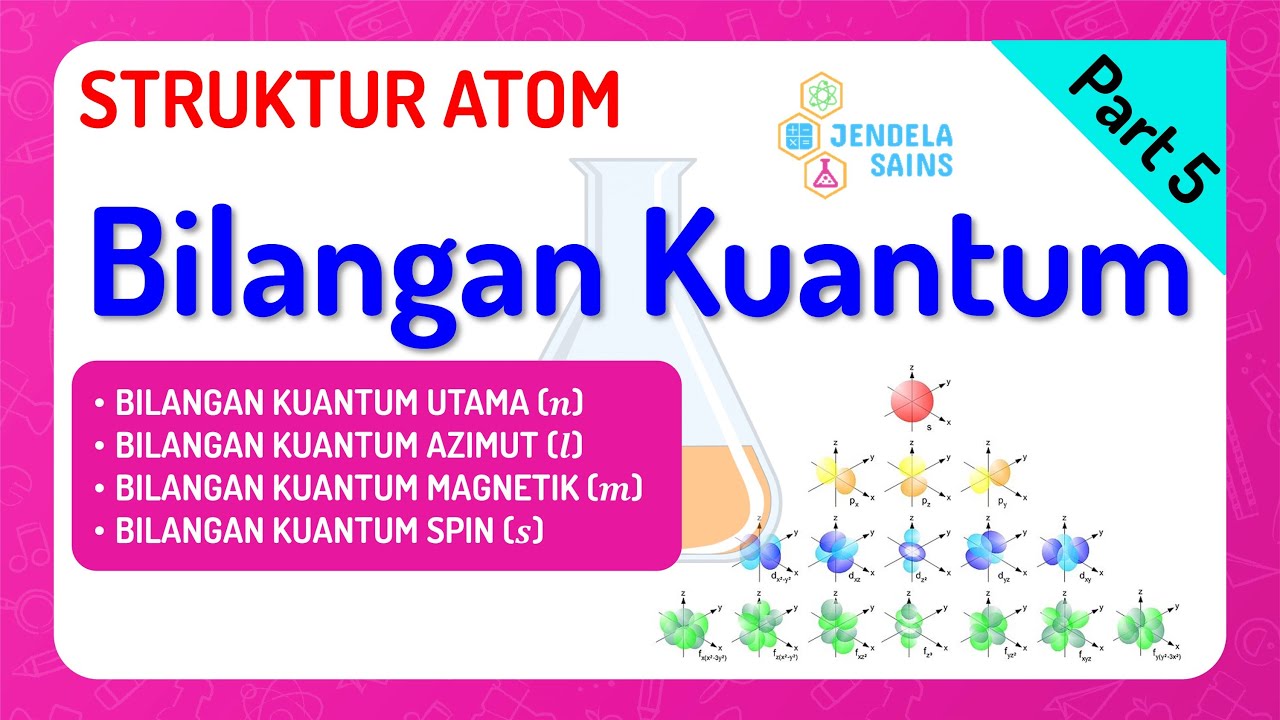
- 😀 The first quantum number, the principal quantum number (n), defines the electron's energy level and distance from the nucleus.
- 😀 The second quantum number (L) defines the shape of the orbital, such as S, P, D, and F orbitals.
- 😀 The 'S' orbital has a spherical shape, while 'P' orbitals have a dumbbell shape with a node at the nucleus.
- 😀 The magnetic quantum number (ML) describes the orientation of the orbital in 3D space.
- 😀 For an S orbital (L=0), ML can only be 0. For a P orbital (L=1), ML can be -1, 0, or +1, which corresponds to three possible orientations.
- 😀 The spin quantum number (ms) describes the intrinsic spin of an electron, which can be either +1/2 or -1/2.
- 😀 The Pauli Exclusion Principle states that no two electrons in the same atom can have the same set of four quantum numbers.
- 😀 The exclusion principle means that each orbital can hold a maximum of two electrons, each with opposite spins.
- 😀 The structure of quantum numbers and the Pauli Exclusion Principle determine how electrons fill orbitals, directly influencing chemical bonding and the periodic table's structure.
Q & A
What is a quantum number in the context of an atom?
-A quantum number is a number used to uniquely describe the properties and location of an electron within an atom, effectively giving it a unique 'address'.
What does the principal quantum number (n) represent?
-The principal quantum number, n, represents the main energy level or shell of an electron. Higher n values correspond to higher energy levels and orbitals farther from the nucleus.
How does the azimuthal (secondary) quantum number (L) relate to the shape of an orbital?
-The azimuthal quantum number, L, determines the shape of the orbital. For example, L = 0 corresponds to an S orbital (spherical), L = 1 to a P orbital (dumbbell-shaped), and higher L values correspond to D and F orbitals.
What is the range of values for L given a principal quantum number n?
-The value of L ranges from 0 to n-1. So, for a given energy level n, L can take any integer value from 0 up to one less than n.
What does the magnetic quantum number (ML) specify?
-The magnetic quantum number, ML, specifies the orientation of an orbital in 3D space. Its possible values range from −L to +L, indicating different possible orientations for the same orbital shape.
Why can an S orbital only have one orientation?
-An S orbital is spherical (L = 0), which looks the same in all directions, so it has only one possible orientation, ML = 0.
What does the spin quantum number (MS) describe?
-The spin quantum number, MS, describes the intrinsic spin of an electron, a fundamental property that acts like a tiny magnet. It can only have two values: +½ (spin up) or −½ (spin down).
What is the Pauli exclusion principle and why is it important?
-The Pauli exclusion principle states that no two electrons in the same atom can have identical sets of all four quantum numbers. It ensures that each electron has a unique 'address,' which determines how electrons fill orbitals and ultimately governs chemical behavior.
How many electrons can occupy a single orbital and why?
-A single orbital can hold a maximum of two electrons because they must have opposite spins to satisfy the Pauli exclusion principle.
How do quantum numbers influence the structure of the periodic table?
-Quantum numbers dictate how electrons fill the available orbitals in atoms, which in turn determines the chemical properties and periodic trends of elements. This filling order is the foundation for the organization of the periodic table.
What is the relationship between an electron's orbital and nodes?
-Nodes are regions in an orbital where the probability of finding an electron is zero. For example, P orbitals have a node at the nucleus, meaning the electron is never found exactly there.
How do nested dependencies work among the first three quantum numbers?
-The quantum numbers are interdependent: L depends on n (0 ≤ L ≤ n−1), and ML depends on L (−L ≤ ML ≤ +L). This nested structure determines orbital shapes, orientations, and energy levels in a hierarchical way.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahora5.0 / 5 (0 votes)