BILANGAN KUANTUM
Summary
TLDRIn this educational video, Udin explains the concept of quantum numbers, which are used to describe the energy levels, orbitals, and positions of electrons in an atom. The video covers the four main quantum numbers: principal (n), azimuthal (l), magnetic (m), and spin (s). Udin provides clear examples using atoms with different electron configurations, demonstrating how to determine the quantum numbers for both the last and outermost electrons. The video aims to make this complex topic accessible and engaging through simple explanations and practical examples.
Takeaways
- 😀 Quantum numbers describe the energy level and position of electrons within an atom based on quantum mechanics.
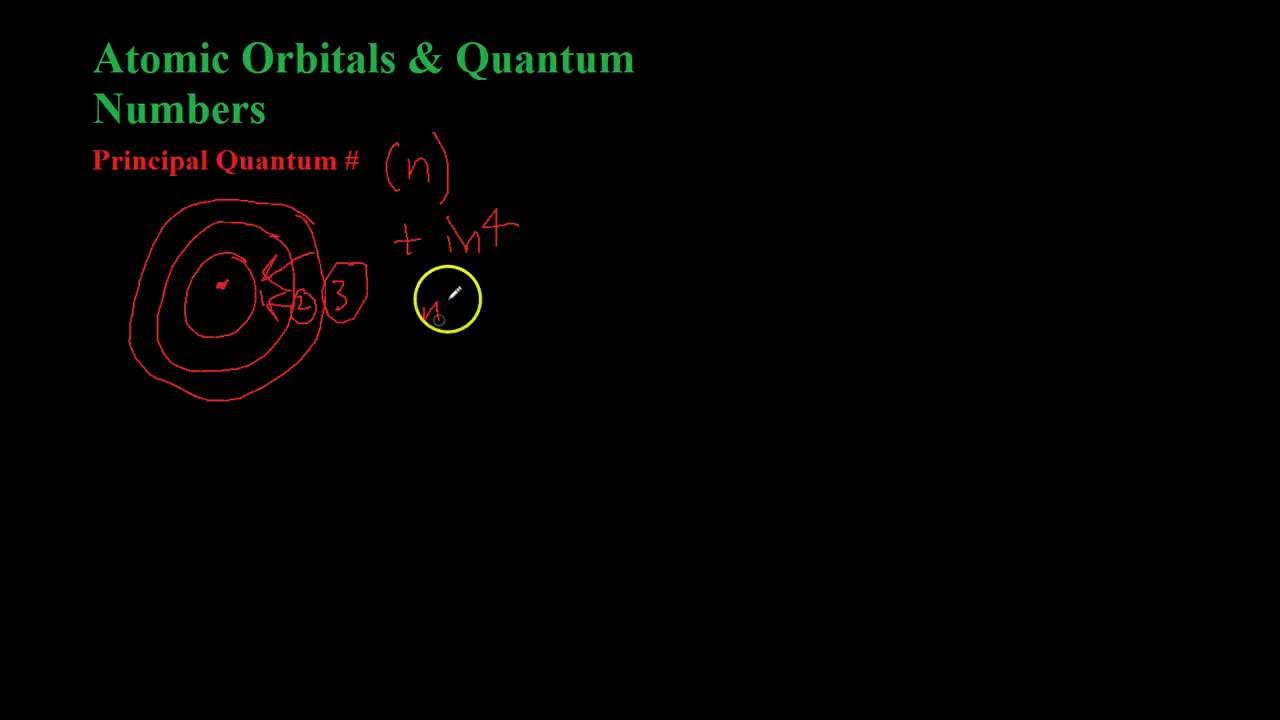
- 😀 The first type of quantum number is the principal quantum number (n), which indicates the energy shell or level of the electron.
- 😀 The azimuthal quantum number (l) defines the shape or type of orbital, such as s, p, d, or f orbitals.
- 😀 The magnetic quantum number (m) specifies the orientation of the orbital in space.
- 😀 The spin quantum number (s) represents the direction of electron spin, either +1/2 or -1/2.
- 😀 The principal quantum number (n) can take integer values starting from 1 and increases with the energy level.
- 😀 The azimuthal quantum number (l) depends on n and ranges from 0 to (n-1), with specific values for each orbital type: s (l=0), p (l=1), d (l=2), and f (l=3).
- 😀 The magnetic quantum number (m) ranges from -l to +l, providing the possible orientations of the orbital.
- 😀 The spin quantum number (s) indicates the direction of the electron’s rotation, with possible values of +1/2 or -1/2.
- 😀 Understanding quantum numbers helps determine the electron configuration and predict electron behavior in atoms.
Q & A
What are quantum numbers in chemistry?
-Quantum numbers are values that describe the energy and position of electrons within an atom. They provide important details about the electron's energy level, orbital shape, orientation, and spin.
What is the first quantum number, and what does it represent?
-The first quantum number is the Principal Quantum Number (n). It represents the main energy level or shell of an electron, which is denoted by a positive integer (1, 2, 3, etc.). The value of n determines the size and energy of the orbital.
What is the second quantum number, and what does it tell us?
-The second quantum number is the Azimuthal Quantum Number (l). It describes the shape of the orbital where the electron is located. The values of l range from 0 to (n-1) and correspond to the types of orbitals: s (l=0), p (l=1), d (l=2), and f (l=3).
What is the third quantum number, and how is it defined?
-The third quantum number is the Magnetic Quantum Number (m). It represents the orientation of the orbital in space. The value of m ranges from -l to +l, depending on the value of the azimuthal quantum number (l).
What does the fourth quantum number, spin (s), signify?
-The fourth quantum number is the Spin Quantum Number (s). It indicates the direction of the electron's spin, which can either be +1/2 (clockwise) or -1/2 (counterclockwise).
How are quantum numbers used to determine the electron configuration of an atom?
-Quantum numbers are used to describe each electron's position within an atom. By using these numbers, we can determine the electron configuration, which tells us how electrons are arranged in various orbitals and energy levels.
How do you calculate the quantum numbers for an atom with atomic number 26?
-For an atom with atomic number 26, we first look at the electron configuration, which is [Ar] 4s² 3d⁶. The quantum numbers for the last electron (in the 3d orbital) would be: n = 3, l = 2, m = -2, and s = -1/2. The outermost electron is in the 4s orbital with n = 4, l = 0, m = 0, and s = -1/2.
What is Hund's Rule, and how does it apply to quantum numbers?
-Hund's Rule states that when electrons fill degenerate orbitals (orbitals of the same energy level), they first occupy separate orbitals with parallel spins before pairing up. This ensures that the maximum number of unpaired electrons is achieved. In quantum numbers, this rule helps determine the values for the magnetic quantum number (m) and spin (s).
What is the process to determine the quantum numbers of the last electron in an atom?
-To determine the quantum numbers of the last electron, you first write the electron configuration for the atom, identify the last electron (which fills the highest energy orbital), and then assign the quantum numbers based on its position in the orbital, energy level, and spin direction.
What are the possible values for the magnetic quantum number (m) when l = 2?
-When l = 2 (for a d orbital), the magnetic quantum number (m) can take values ranging from -2 to +2. Therefore, m can be -2, -1, 0, +1, or +2.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Cara Mudah‼️Menentukan Bilangan Kuantum (Utama, Azimut, Magnetik, Spin)
4.2 Quantum Model of the Atom

3.5.3 - Composição de um orbital atômico: Número quântico principal (camada ou nível de energia)

BILANGAN KUANTUM

THE QUANTUM MECHANICAL MODEL OF THE ATOM | GRADE 9 SCIENCE QUARTER 2 MODULE 1 LESSON 1 TAGALOG

BILANGAN KUANTUM
5.0 / 5 (0 votes)