Termologia | Calorimetria - Parte II (RESUMÃO)
Summary
TLDRThis video lesson covers key concepts of calorimetry, focusing on heat curves, phase transitions, and formulas for heat transfer. The teacher explains how heat affects the temperature and state of a substance, from solid to liquid to gas, and introduces essential formulas for calculating sensible and latent heat. The lesson also covers heat transfer between bodies, emphasizing thermal equilibrium, and provides practical examples for solving calorimetry problems. The concept of power in calorimetry is explored, with a focus on its importance in exams like the vestibular and ENEM. This lesson is a comprehensive guide to understanding heat and thermodynamic calculations.
Takeaways
- 😀 Understanding the heating curve is essential for calculating heat transfer during temperature changes and phase transitions.
- 😀 The heating curve represents a substance transitioning from solid to liquid (fusion) and from liquid to gas (vaporization), with no temperature change during phase changes.
- 😀 To calculate the heat involved in each stage of the heating process, we use formulas for sensible heat (MCΔT) and latent heat (ML).
- 😀 Sensible heat involves a temperature change and is calculated using the formula MCΔT, where M is mass, C is specific heat capacity, and ΔT is the temperature change.
- 😀 Latent heat occurs during phase changes, such as fusion and vaporization, where temperature does not change but energy is absorbed or released.
- 😀 For phase changes, use the formulas Q = ML for fusion (solid to liquid) and Q = ML for vaporization (liquid to gas), where L is the latent heat.
- 😀 The formula Q = MCΔT can be used to calculate sensible heat when temperature changes but there is no phase change.
- 😀 In a closed, isolated system, the total heat exchange is zero, meaning heat lost by one body is gained by another body (Q = -Q).
- 😀 In a non-isolated system, the heat received by the system is not equal to the heat lost, allowing for heat exchange with the surroundings.
- 😀 Power (P) is defined as the rate at which heat is transferred, calculated as P = Q / Δt, where Q is heat and Δt is time, and can be measured in calories per minute or joules per second (watt).
Q & A
What is the concept of a heating curve in calorimetry?
-A heating curve in calorimetry shows how the temperature of a substance changes as it absorbs heat. Initially, the temperature increases until the substance reaches its melting point, then remains constant during phase changes (such as melting or boiling), and finally increases again once the substance is completely in the new phase.
What is the significance of the melting point in a heating curve?
-The melting point is the temperature at which a solid changes to a liquid. During this phase change, even though heat is added, the temperature does not increase until the entire substance has melted.
What happens when a substance reaches the boiling point on the heating curve?
-When a substance reaches the boiling point, it begins to undergo vaporization. Similar to melting, the temperature does not increase during this phase change until all of the substance has vaporized into gas.
How do you calculate the heat involved in a phase change, such as melting or vaporization?
-For phase changes, the heat involved is calculated using the latent heat formula: Q = m * L, where Q is the heat added, m is the mass of the substance, and L is the latent heat of fusion (for melting) or latent heat of vaporization (for boiling).
What is the difference between sensible heat and latent heat?
-Sensible heat refers to the heat that causes a temperature change in a substance, while latent heat refers to the heat required to change the phase of a substance without changing its temperature.
What is the formula for calculating the heat involved in temperature changes (sensible heat)?
-The formula for sensible heat is Q = m * c * ΔT, where Q is the heat added, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
What is the concept of thermal equilibrium in heat exchange?
-Thermal equilibrium occurs when two bodies in contact reach the same temperature, resulting in no net heat flow between them. Heat will flow from the hotter body to the cooler one until they equalize.
What is the equation used to express heat exchange in an isolated system?
-In an isolated system, the total heat exchanged is zero. This is expressed as Q_A + Q_B = 0, where Q_A is the heat lost by one body and Q_B is the heat gained by another body.
How does heat exchange differ in an isolated system compared to a non-isolated system?
-In an isolated system, no heat is exchanged with the surroundings, meaning the heat lost by one body is gained by another. In a non-isolated system, heat can be exchanged with the environment, and the system may lose or gain heat from outside.
What is the formula for calculating power in calorimetry?
-Power is calculated by the formula P = Q / Δt, where P is the power, Q is the heat transferred, and Δt is the time interval over which the heat transfer occurs.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados
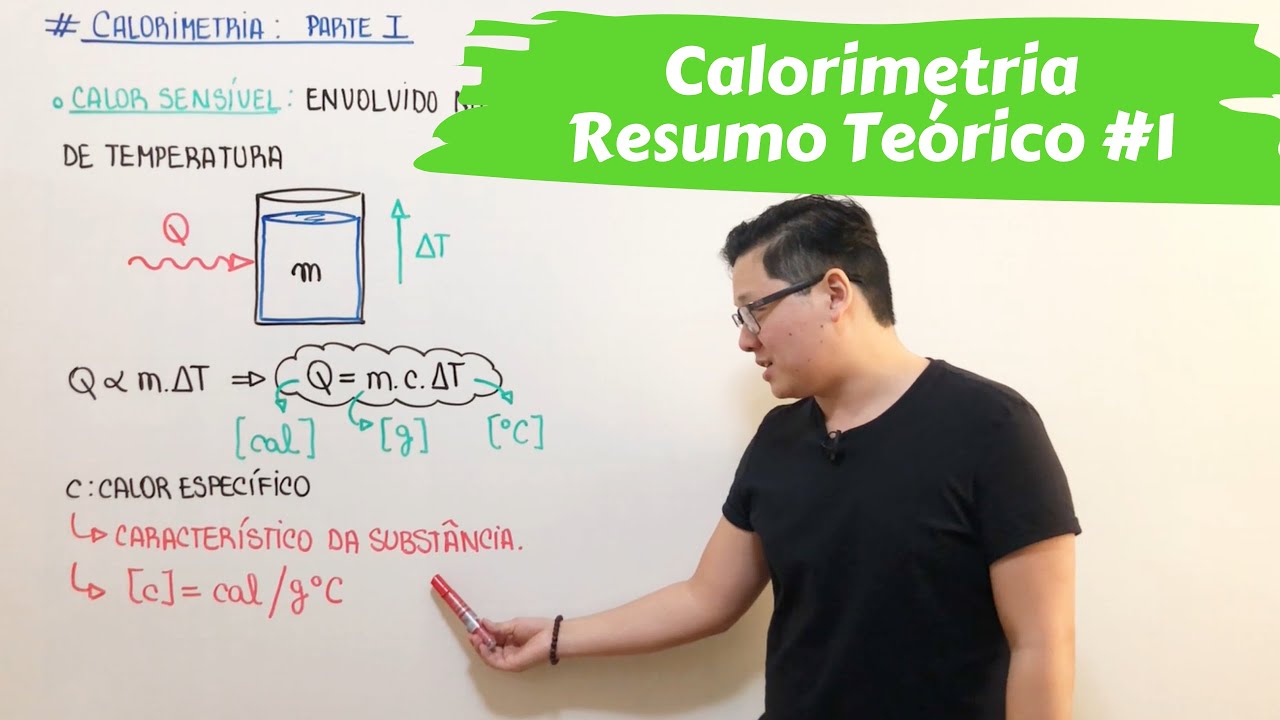
Termologia | Calorimetria - Parte I (RESUMÃO)
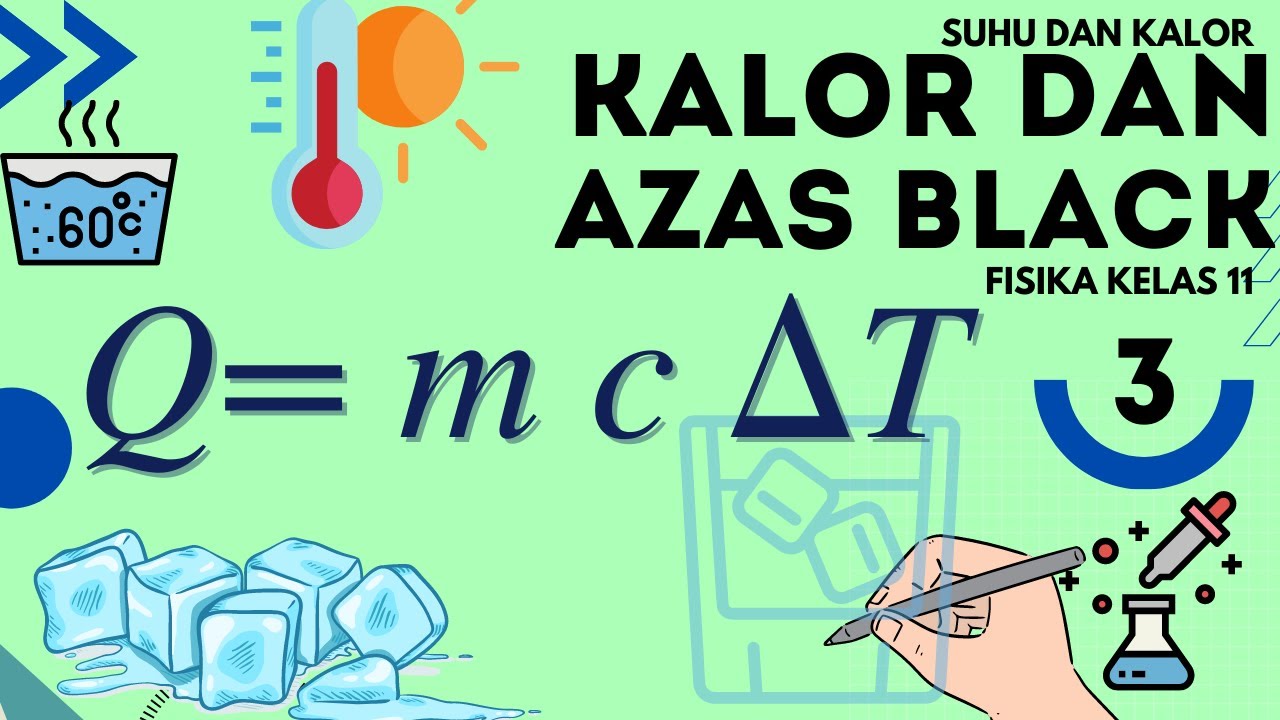
Suhu dan Kalor Fisika Kelas 11 - Part 3 : Kalor dan Azas Black

KALOR | Suhu dan Kalor - Fisika Kelas 11

FISIKA KELAS XI | SUHU DAN KALOR (PART 2) - KALOR DAN PENGARUHNYA

TROCAS DE CALOR SEM MUDANÇA DE FASE - TERMOLOGIA - Aula 7 - Prof. Boaro

Kalor dan Asas Black | Rumus Dasar | IPA SMP
5.0 / 5 (0 votes)
