Pengoperasian Spektrofotometer Serapan Atom (SSA)
Summary
TLDRThis video provides a comprehensive overview of operating the SSA (Spectroscopic Atomic Absorption) instrument for chemical analysis. It covers the setup process, from igniting the burner and lamp to calibrating the instrument with blank and standard samples. The script also explains how to perform data analysis in Excel, including creating a regression model and calculating sample concentrations using the Lambert-Beer law. Ideal for those learning about SSA, this video simplifies the process and demonstrates how to obtain accurate sample measurements and analyze the results effectively.
Takeaways
- 😀 The SSA instrument is used for chemical analysis by measuring the absorbance of samples.
- 😀 Asetonitril gas is used in the SSA to create a flame that excites electrons in the sample for analysis.
- 😀 Samples must be in solution form and free of precipitates to avoid burner clogging.
- 😀 The SSA instrument is controlled through a computer interface, which allows for adjustments in settings like flame and lamp intensity.
- 😀 A blank sample and standard samples of known concentrations are used for calibration before analyzing unknown samples.
- 😀 Absorbance readings should be taken multiple times to ensure accuracy in measurement.
- 😀 After completing the sample analysis, the SSA instrument and computer should be shut down properly to prevent errors.
- 😀 Data analysis is performed using Excel by plotting the concentration of standard samples (x-axis) against their absorbance (y-axis).
- 😀 A linear regression analysis in Excel helps generate a calibration curve, allowing the calculation of unknown sample concentrations based on their absorbance.
- 😀 The regression equation derived from the calibration curve is used to find the concentration of unknown samples from their absorbance values.
- 😀 The script also provides a specific example of calculating the concentration of a sample with a given absorbance of 0.37, using the regression equation.
Q & A
What is the purpose of the SSA instrument explained in the video?
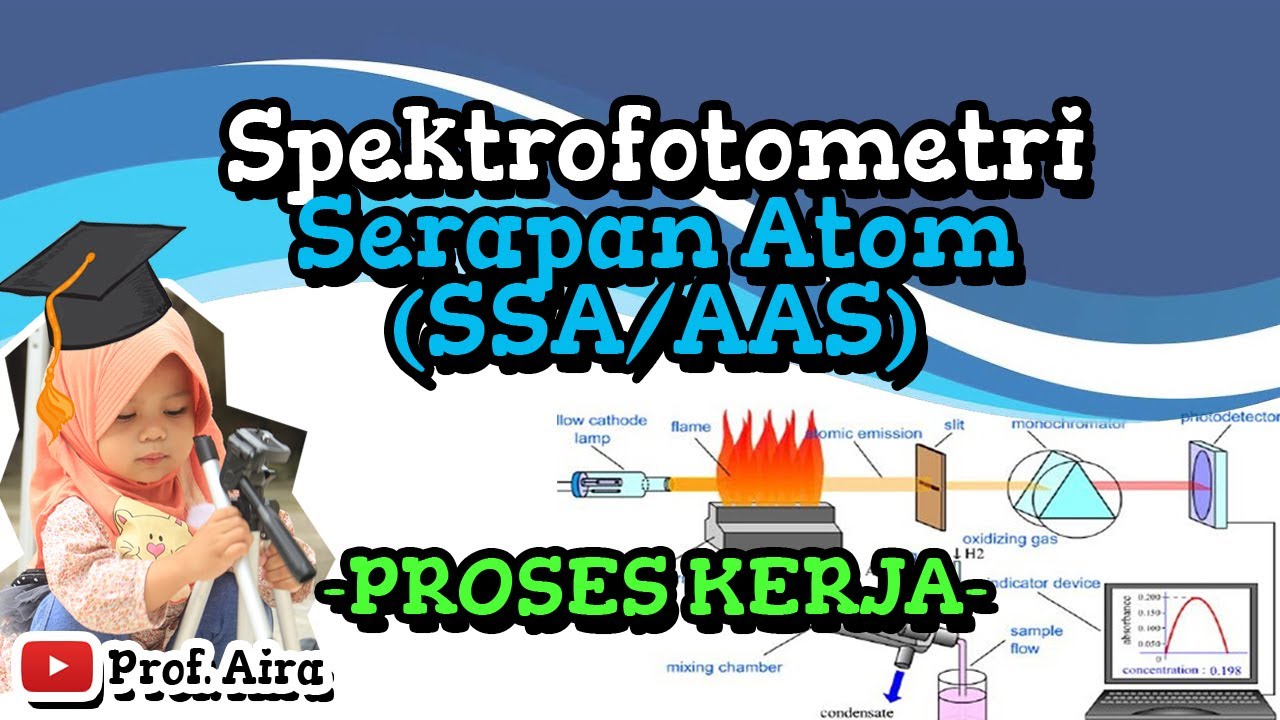
-The SSA (Spectral Scanning Absorbance) instrument is used to analyze the concentration of samples through absorbance measurements. It uses a gas flame and light excitation to excite electrons in the sample, which helps to measure absorbance and determine concentrations.
What role does acetone nitrile gas play in the SSA instrument?
-Acetone nitrile gas serves as the burner fuel in the SSA instrument. It is carried into the burner where it is ignited, producing a flame used for exciting the sample's electrons in the analysis process.
Why is it important to use a liquid sample without sediment in SSA analysis?
-Using a liquid sample without sediment is crucial because any sediment could cause buildup or residue on the burner, which may interfere with the analysis process and the accurate measurement of absorbance.
What functions can the computer connected to the SSA instrument control?
-The computer manages various aspects of the SSA instrument, including controlling the flame, light source, absorbance readings, and the number of repetitions for measurements. It also records and processes the data.
What is the purpose of the blank sample in the SSA analysis process?
-The blank sample is used to calibrate the SSA instrument by setting the baseline absorbance value to zero. This ensures that the subsequent readings of the sample are accurate and not influenced by any background absorbance.
How is the concentration of a sample calculated in the SSA method?
-The concentration of a sample is determined using the Beer-Lambert Law, which relates absorbance (Y) to concentration (X) through a linear regression equation. The sample's absorbance value is used to calculate its concentration by comparing it to the calibration curve created from standard solutions.
What is the purpose of performing a linear regression in the SSA data analysis?
-Linear regression is used to establish a mathematical relationship between absorbance and concentration. By plotting standard concentration values against their corresponding absorbance values, a regression equation can be derived to calculate the concentration of unknown samples.
What does the term 'slope' refer to in the linear regression equation for SSA analysis?
-In the linear regression equation, the 'slope' (denoted as 'A') represents the rate at which absorbance changes with respect to concentration. It is used to calculate the concentration of unknown samples by relating their absorbance values to the slope.
Why is it important to turn off the SSA instrument components, such as the lamp and computer, after completing the analysis?
-Turning off the components after completing the analysis ensures that the instrument is properly shut down, preventing unnecessary energy use and extending the lifespan of the equipment. It also ensures that the data processing is completed and the system is reset for future use.
What should be done if the absorbance readings do not match the expected values after using the SSA instrument?
-If the absorbance readings are incorrect or do not match expectations, it may indicate an issue with the lamp or the calibration. In such cases, the lamp should be replaced, and the instrument should be recalibrated to ensure accurate results.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

Lead Detection Using Flame AA Spectroscopy
SPEKTROFOTOMETRI SERAPAN ATON (SSA/AAS) – PROSES KERJA

Espectrofotômetro Manual - Ajuda Galeno!

UAD - Kuliah Online 1475530 Karakterisasi Material Lanjut (Lecture 2a - part 1)

Spektrometri NMR (Pengenalan & Prinsip Kerja)

Sistem pencernaan Manusia : Struktur dan fungsi sistem pencernaan makanan pada manusia
5.0 / 5 (0 votes)
