Physical vs Chemical Properties
Summary
TLDRThis video explains the difference between physical and chemical properties. Physical properties, like boiling point, melting point, and color, do not alter a substance’s chemical identity, while chemical properties, such as flammability and reactivity, involve a change in the substance’s composition. The video covers various examples, such as the physical property of ductility in metals and the chemical property of combustibility in gasoline, to clarify how these properties are identified. The goal is to help viewers understand how substances behave and react in different contexts, distinguishing physical changes from chemical reactions.
Takeaways
- 😀 Physical properties describe characteristics of a substance that do not change its chemical identity. Examples include boiling point, melting point, and viscosity.
- 😀 Chemical properties describe characteristics that result in a change in the chemical identity of a substance. Examples include flammability, corrosiveness, and combustibility.
- 😀 Boiling point is a physical property because it involves a change in the state of matter (liquid to gas) without altering the chemical identity of a substance (e.g., water remains H2O).
- 😀 Melting point and freezing point are physical properties as they describe changes in state (solid to liquid or liquid to solid) without altering the chemical composition.
- 😀 Flammability is a chemical property because burning a substance (e.g., paper) causes a chemical reaction, producing new substances like carbon dioxide and water.
- 😀 Ductility, which is the ability to stretch a material into wire (e.g., copper), is a physical property because it does not change the chemical identity of the material.
- 😀 Corrosiveness is a chemical property, as it refers to the ability of a substance (like acid) to react with another substance (e.g., metal) and alter its chemical composition.
- 😀 Malleability, or the ability to shape a material (e.g., aluminum), is a physical property because the chemical identity of the material does not change.
- 😀 Explosiveness is a chemical property, as it involves a chemical reaction (e.g., TNT reacting with itself) that produces new substances or energy.
- 😀 pH is a chemical property because it relates to how a substance reacts with others, especially in the case of acids and bases, influencing chemical behavior and reactions.
Q & A
What is the main difference between physical and chemical properties?
-Physical properties describe characteristics that do not change the chemical identity of a substance, while chemical properties describe characteristics that result in a change to the chemical identity of the substance.
What is an example of a physical property?
-An example of a physical property is the boiling point of water. The boiling point is a temperature at which water changes from a liquid to a gas, but its chemical identity (H₂O) remains unchanged.
How is boiling point related to physical properties?
-Boiling point is a physical property because it describes the temperature at which a substance changes state (liquid to gas) without altering its chemical composition.
Is flammability a physical or chemical property?
-Flammability is a chemical property because it involves a chemical reaction that results in the formation of new substances, like when paper burns and turns into carbon dioxide and water.
What happens when a substance is flammable?
-When a substance is flammable, it can burn in the presence of oxygen, undergoing a chemical reaction that changes its chemical identity and forms new substances.
What is an example of a substance with a physical property of ductility?
-Copper is an example of a substance with the physical property of ductility. Copper can be pulled into a wire, but its chemical identity remains unchanged during the process.
What does it mean if a substance is described as corrosive?
-If a substance is corrosive, it means it can cause a chemical reaction that breaks down other materials, often by stripping electrons from metals. This is a chemical property.
How does color relate to physical properties?
-Color is a physical property because it is an observable characteristic of a substance that does not involve a chemical change. However, a color change can indicate a chemical reaction has occurred.
What is the role of pH in distinguishing between physical and chemical properties?
-pH is a chemical property because it describes how acidic or basic a substance is and influences how it reacts with other substances, such as metals or bases.
Why is viscosity considered a physical property?
-Viscosity is considered a physical property because it describes how easily a fluid flows, which is a characteristic that does not involve a chemical change. For example, syrup has higher viscosity than water.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

General Chemistry 1 - Matter and Its Properties

Intramolecular vs. Intermolecular forces - London Dispersion, Dipole-Dipole, Ion-Dipole forces -Chem
SIFAT DAN PERUBAHAN ZAT | SIFAT FISIKA DAN KIMIA | PERUBAHAN FISIKA DAN KIMIA

Physical vs Chemical Properties - Explained

Fuels| Introduction, Classification and Properties |Dr. Anjali Ssaxena
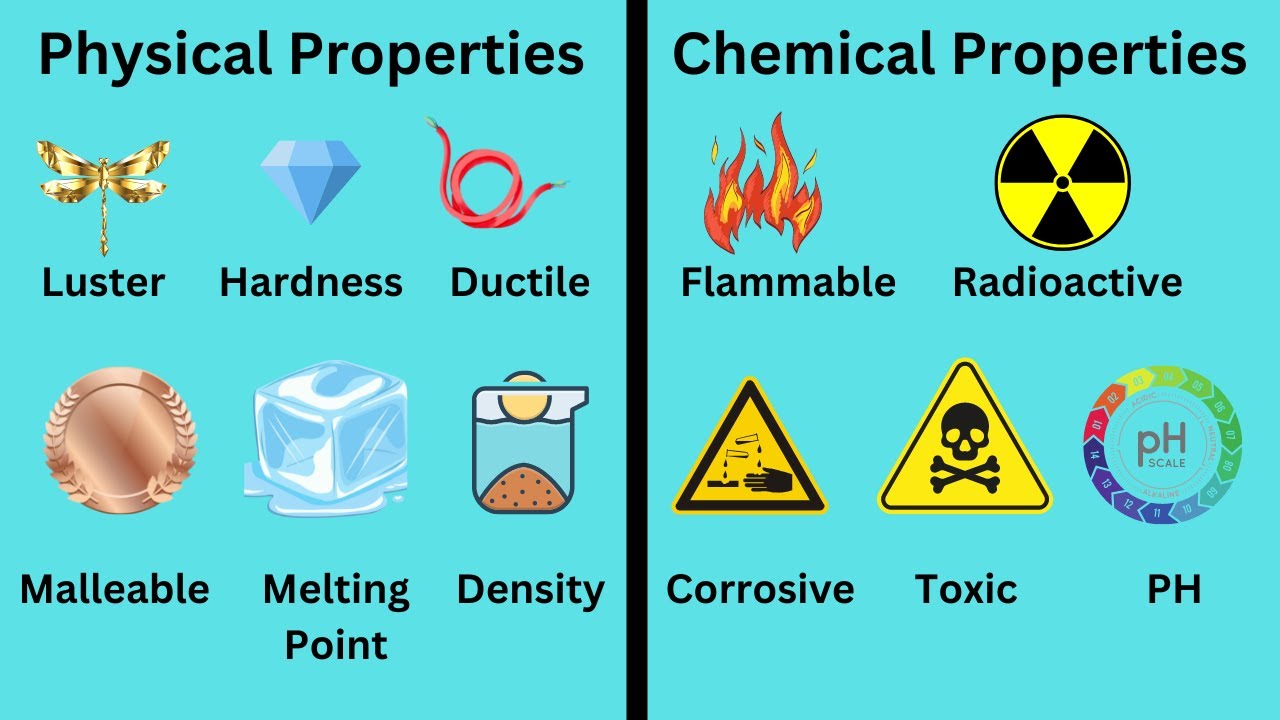
Physical and Chemical Properties
5.0 / 5 (0 votes)
