What Are Structural Isomers | Organic Chemistry | Chemistry | FuseSchool
Summary
TLDRThis video explains the concept of structural isomers in alkanes, showcasing how molecules with the same molecular formula can have different structural arrangements. Through examples of methane, ethane, propane, butane, octane, and hexane, viewers learn how the longest carbon chain and attached groups determine the compound's name. It highlights the historical use of octane isomers in leaded petrol and explores the environmental impact of leaded fuel. The video also encourages viewers to identify structural isomers through exercises, emphasizing the importance of molecular structure in chemical naming and function.
Takeaways
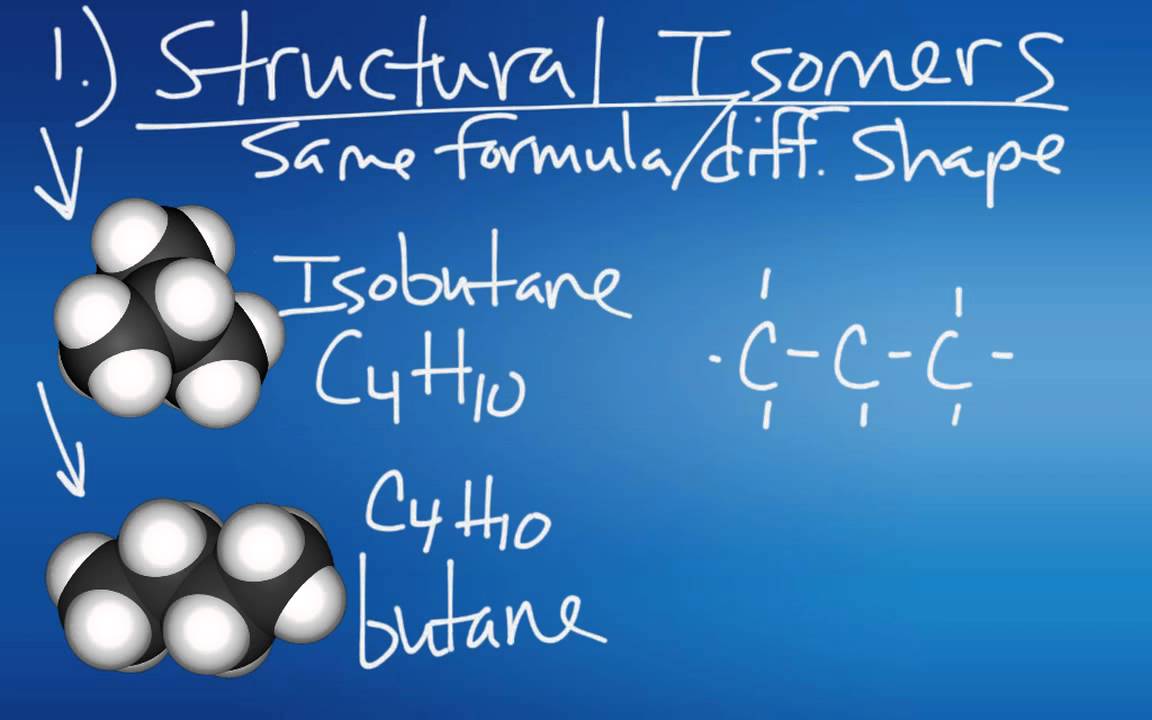
- 😀 Structural isomers are compounds that have the same molecular formula but differ in their atomic arrangement.
- 😀 Alkanes are hydrocarbons that consist of chains of carbon atoms, with hydrogen atoms bonded to them to satisfy carbon's valency.
- 😀 The naming of structural isomers is based on the longest carbon chain and the position of side groups like methyl or ethyl groups.
- 😀 Butane (C₄H₁₀) has two structural isomers: a straight chain (butane) and a branched chain (2-methylpropane).
- 😀 The longest chain in an isomer determines its base name (e.g., propane for a three-carbon chain, pentane for a five-carbon chain).
- 😀 The 1920s discovery of octane isomers helped create smoother-burning petrol, which reduced engine knocking.
- 😀 Lead-based compounds like tetraethyl lead were used in petrol to improve combustion efficiency but were later banned due to their toxic effects.
- 😀 In the case of hexane (C₆H₁₄), there are five isomers, including variations based on pentane and butane structures.
- 😀 The formal names of hexane isomers are based on the length of the longest chain and where methyl or ethyl groups are attached (e.g., 2-methylpentane, 3-methylpentane).
- 😀 The story of lead-free petrol highlights the importance of understanding molecular structures and their applications in real-world industries like automotive fuel production.
- 😀 Structural isomers, despite having the same number of atoms, differ significantly in their chemical properties due to their distinct arrangements.
Q & A
What are structural isomers?
-Structural isomers are compounds that have the same molecular formula but differ in the arrangement of their atoms. This difference in structure leads to variations in the properties of the molecules.
How are the structural isomers of butane different?
-The two structural isomers of butane (C₄H₁₀) are a straight-chain molecule and a branched-chain molecule. The straight-chain isomer has all four carbon atoms in a continuous chain, while the branched form has three carbon atoms in the longest chain with a methyl group attached to the second carbon atom.
What is the name of the branched isomer of butane?
-The branched isomer of butane is called **2-methylpropane**, where the longest chain consists of three carbon atoms and a methyl group is attached to the second carbon atom.
Why is it important to understand structural isomers in chemistry?
-Understanding structural isomers is important because they have the same molecular formula but can have different chemical and physical properties due to the differences in their atomic arrangement. This can affect their reactivity, boiling points, solubility, and other characteristics.
How did leaded petrol affect the environment?
-Leaded petrol, which contained tetraethyl lead, helped engines burn fuel more smoothly but resulted in toxic lead emissions. Lead oxide formed in the exhaust, contaminating the air, soil, and even ice in Antarctica, leading to widespread environmental pollution that persisted for decades.
What was the role of tetraethyl lead in petrol?
-Tetraethyl lead was added to petrol to improve engine performance by reducing knocking. It made the petrol burn more smoothly, but it also introduced a toxic substance into the environment, which led to the phase-out of leaded petrol over time.
What are the isomers of octane, and why were they important?
-Octane (C₈H₁₈) has several structural isomers. One important isomer is **3-ethyl-2-methylpentane**, which was used to improve the combustion properties of petrol. Isomers like this helped reduce engine knocking and made petrol more efficient for car engines.
What was the significance of the discovery of lead in petrol in 1923?
-In 1923, the discovery that adding lead compounds, like tetraethyl lead, to petrol improved engine performance by reducing knocking was significant. However, the long-term environmental and health consequences of lead pollution were not immediately understood.
How do we name structural isomers?
-Structural isomers are named based on the length of the longest carbon chain and the position of any attached groups, such as methyl or ethyl groups. For example, a compound based on pentane with a methyl group attached to the second carbon would be named **2-methylpentane**.
What are the isomers of hexane, and how are they derived?
-Hexane (C₆H₁₄) has several isomers, including **2-methylpentane**, **3-methylpentane**, **2,3-dimethylbutane**, and **2,2-dimethylbutane**. These isomers are derived by branching the main carbon chain, either from a pentane or butane structure, resulting in different compounds with the same molecular formula.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahora5.0 / 5 (0 votes)