Termodinamika: Sistem terisolasi, tertutup, dan terbuka
Summary
TLDRThis lecture on physical chemistry, aimed at pharmacy students, explores thermodynamics by examining three types of systems: isolated, closed, and open. Isolated systems do not exchange energy or matter with their environment, while closed systems allow energy exchange but not matter. Open systems permit both energy and matter transfer. Using gas systems as examples, the lecture illustrates how temperature differences affect energy transfer. The connection to kinetic theory emphasizes that average kinetic energy correlates with temperature, highlighting the dynamics of energy interactions in various thermodynamic contexts.
Takeaways
- 😀 Understanding the music industry is crucial for artists to navigate their careers effectively.
- 🎶 Technology has transformed how music is produced, distributed, and consumed, offering both challenges and opportunities.
- 💡 Artists must leverage social media platforms to build their brands and connect with audiences directly.
- 📊 The impact of streaming services has reshaped revenue models in the music industry, requiring artists to adapt their strategies.
- 🤝 Collaboration among artists, producers, and technology professionals is essential for innovation and success.
- 🎤 Artist empowerment is increasing, with more independent musicians gaining control over their work and profits.
- 🌍 Globalization of music has expanded market access, allowing artists to reach international audiences.
- 📈 Data analytics plays a significant role in understanding audience preferences and shaping marketing strategies.
- 🎧 The rise of virtual concerts and online performances presents new avenues for artists to engage with fans.
- 🚀 Embracing change and being adaptable are vital traits for artists to thrive in the evolving music landscape.
Q & A
What are the three types of systems discussed in thermodynamics?
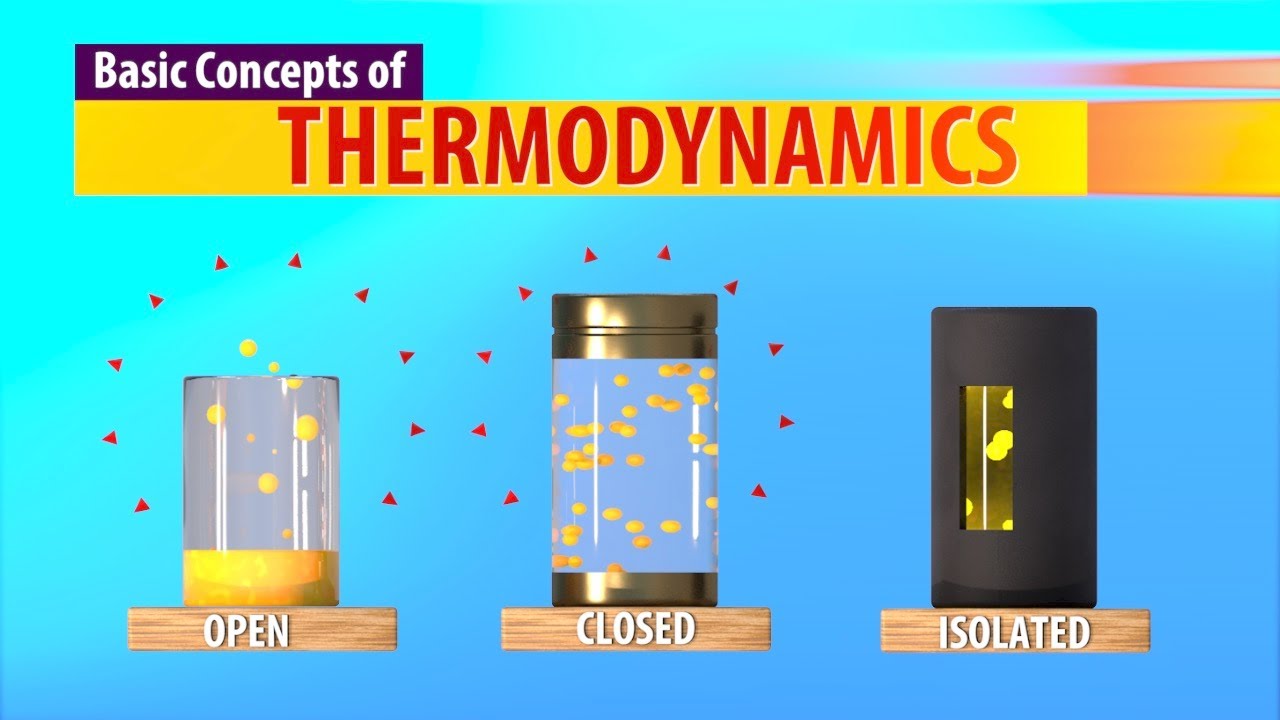
-The three types of systems are isolated systems, closed systems, and open systems, each categorized based on their interactions with the environment regarding energy and matter exchange.
What characterizes an isolated system?
-An isolated system does not exchange energy or matter with its surroundings, meaning nothing can enter or exit the system.
What is the function of an adiabatic wall in an isolated system?
-An adiabatic wall prevents the transfer of energy and matter between the system and the environment, ensuring the system remains isolated.
How does a closed system differ from an isolated system?
-A closed system allows energy exchange with the environment but does not permit matter exchange, meaning energy can flow in or out, but the matter inside remains constant.
What is an open system?
-An open system allows both energy and matter to be exchanged with the environment, meaning particles can move in and out freely.
In the context of gas systems, how do particle speeds relate to temperature?
-The average speed of particles in a gas is proportional to its temperature; higher temperatures correspond to higher average particle speeds.
What happens during particle collisions with an adiabatic wall in an isolated system?
-When particles collide with an adiabatic wall, their kinetic energy remains constant, as the wall does not allow energy transfer, only reflecting the particles.
How does temperature difference between a system and its environment affect energy transfer?
-If the environment's temperature is higher than the system's, energy will flow from the environment to the system; conversely, if the system's temperature is higher, energy will flow from the system to the environment.
What occurs in a closed system when energy is exchanged with the environment?
-In a closed system, when energy is exchanged, the kinetic energy of particles can change due to collisions with the walls, resulting in energy transfer that can affect the overall temperature of the system.
What is the significance of understanding these thermodynamic systems?
-Understanding these systems is crucial for analyzing energy interactions and behaviors in physical chemistry, which can have applications in various scientific fields, including pharmacology.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

Hukum Termodinamika, Bagian 1: Energi Dalam dan Hukum Pertama

Sistem Pada Termodinamika dan Contohnya | Sistem Terbuka, Sistem Tertuttup dan Sistem Terisolasi
Basic Concepts of Thermodynamics (Animation)

Thermodynamics Class 11 in 5 Minutes | Chemistry | Quick Revision | NEET, JEE & CBSE |

Termodinamika - Fisika Kelas 11 (Kurikulum 2013 Revisi) - Quipper Video

Termodinamika Kelas XI IPA
5.0 / 5 (0 votes)
