Diagram fase air dan larutan NaOH adalah sebagai A B berikut, P Keterangan: 6 perubahan fase air ...
Summary
TLDRThis video explains the principles of freezing point depression and boiling point elevation through the example of a NaOH solution. It outlines the formulas used for these calculations, emphasizing the van 't Hoff factor and the importance of molality. The freezing point of the NaOH solution is calculated to be -1.2 °C, while the boiling point rises to 100.33 °C, demonstrating how electrolytes affect solution properties. By applying these concepts, viewers gain a deeper understanding of colligative properties in chemistry, particularly regarding ionic compounds.
Takeaways
- 😀 The transcript explains phase diagrams, focusing on freezing point depression and boiling point elevation.
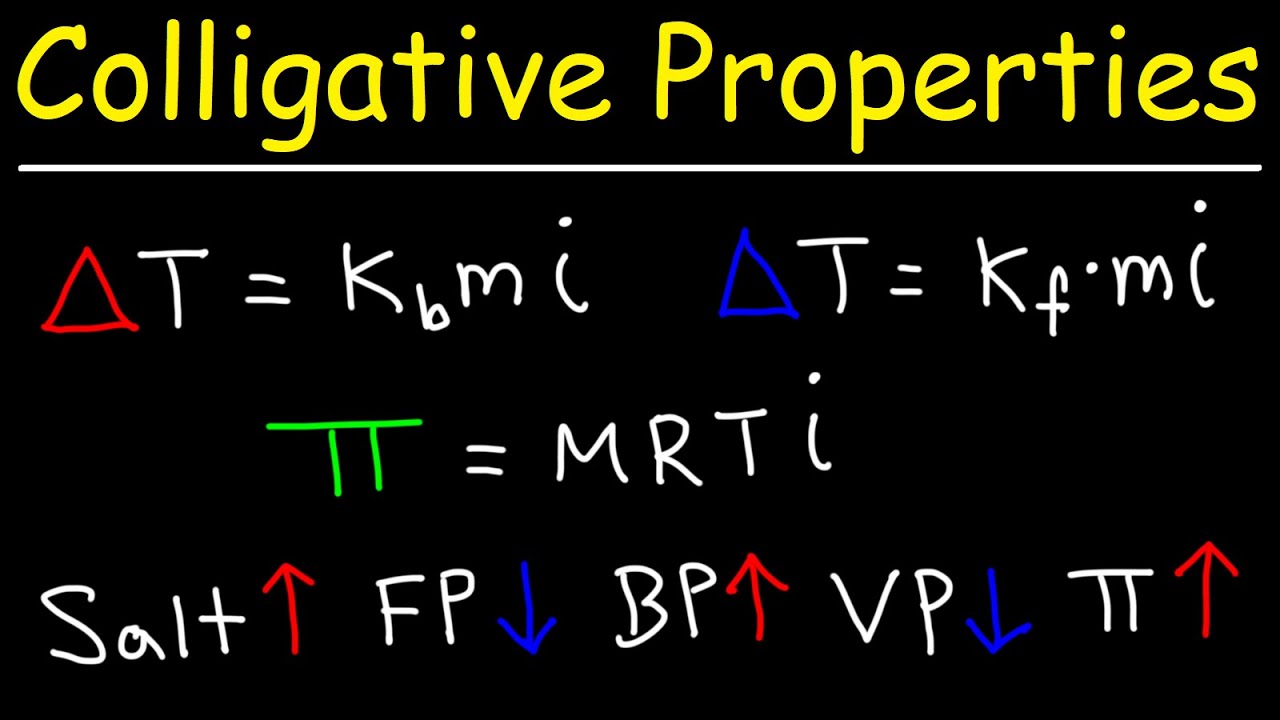
- 📉 The freezing point depression is calculated using the formula ΔT_F = K_F * m * i.
- 📊 The boiling point elevation is calculated using the formula ΔT_B = K_B * m * i.
- 🔍 The Van 't Hoff factor (i) for NaOH is determined to be 2 due to its ionization into Na+ and OH- ions.
- 🧪 The molality of the NaOH solution is calculated to be 0.33 molal based on the freezing point depression.
- ❄️ The freezing point of the solvent (water) is 0°C, leading to a freezing point of -1.2°C for the NaOH solution.
- 🌡️ The boiling point elevation constant (K_B) is given as 0.5, which is crucial for the boiling point calculation.
- 📈 The boiling point of the NaOH solution is found to be 100.33°C after adding the boiling point elevation to the solvent's boiling point.
- 💡 Strong electrolytes like NaOH fully ionize, which affects the calculations of colligative properties.
- 🔗 Understanding these concepts is essential for practical applications in chemistry and real-world scenarios.
Q & A
What is the freezing point depression formula mentioned in the transcript?
-The freezing point depression formula is ΔTF = KF * m * i, where ΔTF is the depression of the freezing point, KF is the freezing point depression constant, m is the molality, and i is the van 't Hoff factor.
How do you calculate the van 't Hoff factor (i) for NaOH?
-For NaOH, the van 't Hoff factor (i) is calculated using the formula i = 1 + α(n - 1), where α is the degree of ionization and n is the number of ions produced. Since NaOH is a strong electrolyte that fully dissociates into Na+ and OH-, i equals 2.
What is the significance of the van 't Hoff factor in colligative properties?
-The van 't Hoff factor is significant in colligative properties as it indicates the number of particles that a solute produces in solution, affecting the calculation of freezing point depression and boiling point elevation.
What are the boiling point elevation and its formula for electrolytic solutions?
-The boiling point elevation is given by the formula ΔTB = KB * m * i, where ΔTB is the elevation of the boiling point, KB is the boiling point elevation constant, m is the molality, and i is the van 't Hoff factor.
What was the molality of the solution calculated in the transcript?
-The molality of the solution calculated in the transcript was 0.33 molal.
What is the boiling point of the solvent (water) mentioned in the script?
-The boiling point of the solvent (water) mentioned in the script is 100 degrees Celsius.
What was the final boiling point of the solution calculated in the transcript?
-The final boiling point of the solution calculated was 100.33 degrees Celsius.
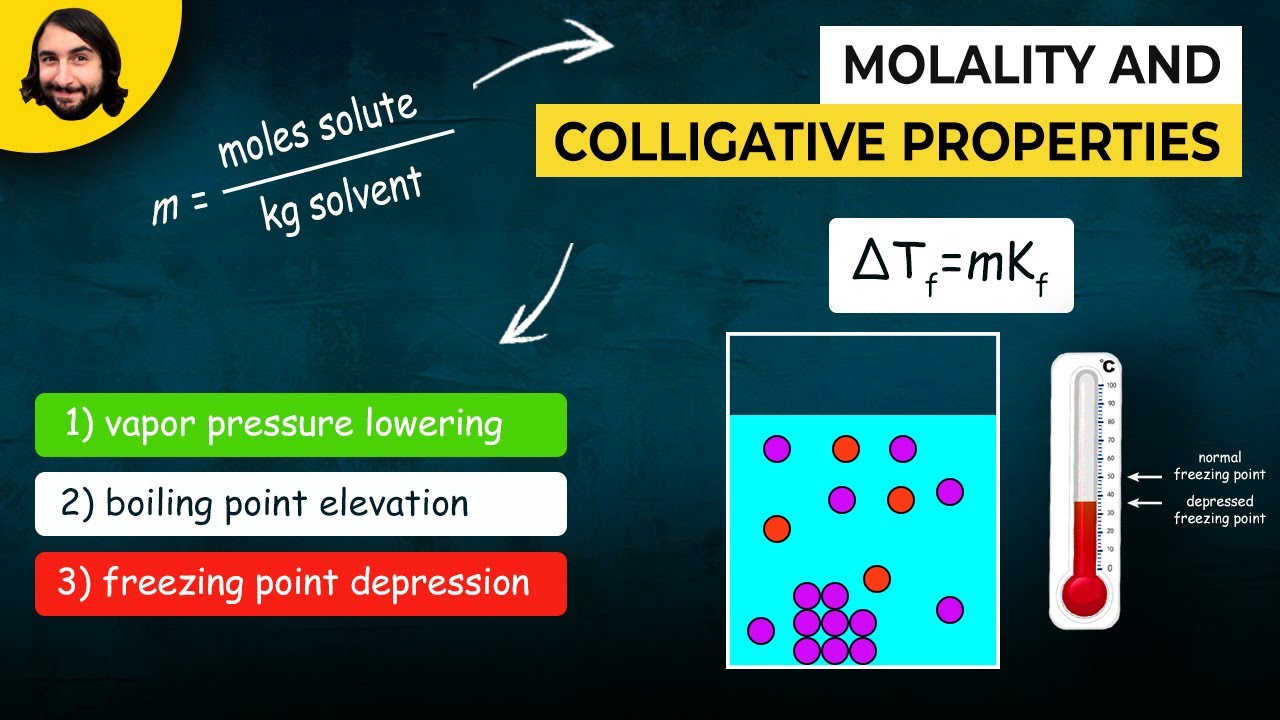
How does the presence of solute affect the freezing point of a solution?
-The presence of a solute lowers the freezing point of a solution compared to the pure solvent, a phenomenon known as freezing point depression.
What is the relationship between molality and the freezing/boiling point changes in solutions?
-Molality is directly related to the freezing and boiling point changes in solutions; higher molality typically results in greater changes in freezing and boiling points due to the increased concentration of solute particles.
Why is NaOH considered a strong electrolyte?
-NaOH is considered a strong electrolyte because it fully dissociates into its constituent ions (Na+ and OH-) in solution, resulting in a complete ionization.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

13.2 Colligative Properties of Solutions (1/2)
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
Molality and Colligative Properties

Sifat Koligatif Larutan -Kimia SMA kelas 12 semester 1

SKL (2)| Kenaikan Titik Didih (∆Tb) | Penurunan Titik Beku (∆Tf)

Química Simples #12 - Resumos - Propriedades Coligativas
5.0 / 5 (0 votes)
